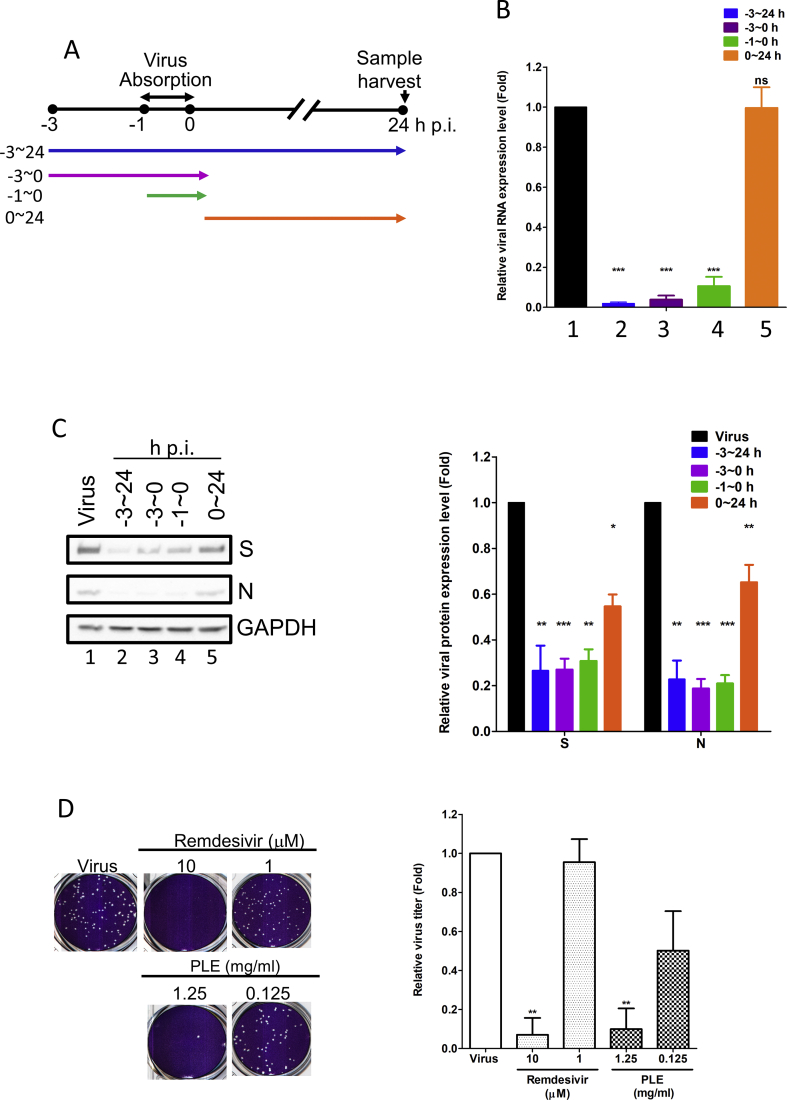
Fig. 1.
Treatment with PLE inhibits SARS-CoV-2 at early stages of replication. (A) Schematic representation of the time-of-addition assay. (B–C) Vero E6 cells were infected with SARS-CoV-2 at a MOI of 0.01. Subsequently, PLE (1.25 mg/mL) was added at the following time points: before virus entry (between −3 and 0 h p.i.), during virus absorption (−1−0 h p.i.), and following virus adsorption (0–24 h p.i.). Infected cells were collectively harvested at 24 h p.i.; viral RNA synthesis and viral protein expression were analyzed with qPCR (B) and western blotting (C), respectively. (B) Expression levels of viral RNA were initially normalized to GAPDH mRNA at each experimental condition. Moreover, the ratio measured in PLE-treated cells was normalized to the RNA level of virus control (arbitrarily set to 1). (C) The intensity of SARS-CoV-2 spike protein (S) and nucleocapsid (N) expression was normalized to GAPDH. Moreover, the ratio measured in PLE-treated cells was normalized to the protein level of virus control (arbitrarily set to 1). N = 3. (D) The results of the plaque reduction assay revealed that SARS-CoV-2 infectivity was diminished after exposure of Vero E6 cells to PLE. SARS-CoV-2 was pre-incubated with various concentrations of PLE or remdesivir before its addition to Vero E6 cells for the plaque assay. The number of plaques was calculated and normalized to that of virus control (arbitrarily set to 1). Data in bar charts are expressed as means ± standard error of the mean from at least two independent experiments. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.005; ns = not significant.