Figure 3.
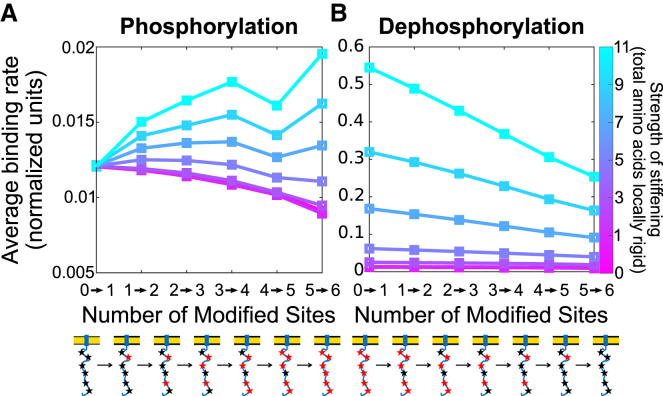
Local stiffening modulates binding rates to multisite disordered domain. (A) Average binding rates of a kinase binding to ζ at different phosphorylation states are shown for varying range of local stiffening (pink: no residues are stiffened; blue: 11 residues are stiffened, five on each side of site). (B) Average binding rates of a phosphatase binding to ζ at different dephosphorylation states are shown for varying ranges of local unstiffening per dephosphorylation event. For both (A) and (B), the schematic below the axis shows example configuration for each phosphorylation state. Kinase and phosphatase radii are 2.1 nm. Rates are normalized to the free-space binding rate . To see this figure in color, go online.