Abstract
The development of safe diagnostic protocols for working with SARS-CoV-2 clinical samples at Biosafety Level 2 (BSL2) requires understanding of the effect of heat-treatment on SARS-CoV-2 viability and downstream RT-PCR sensitivity. In this study heating SARS-CoV-2/England/2/2020 to 56 °C and 60 °C for 15, 30 and 60 min reduced the virus titre by between 2.1 and 4.9 log10 pfu/mL (as determined by plaque assay). Complete inactivation did not occur and there was significant variability between replicates. Viable virus was detected by plaque assay after heat-treatment at 80 °C for 15 or 30 min but not 60 or 90 min. After heat-treatment at 80 °C for 60 min infectious virus was only detected by more sensitive virus culture. No viable virus was detected after heating to 80 °C for 90 min or 95 °C for 1 or 5 min. RT-PCR sensitivity was not compromised by heating to 56 °C and 60 °C. However, RT-PCR sensitivity was reduced (≥3 Ct value increase) after heating the virus to 80 °C for 30 min or longer, or 95 °C for 1 or 5 min. In summary we found that the efficacy of heat-inactivation varies greatly depending on temperature and duration. Local validation of heat-inactivation and its effects downstream is therefore essential for molecular testing.
Keywords: SARS-CoV-2, Heat inactivation
1. Introduction
The current pandemic of SARS-CoV-2 has led to an unprecedented global expansion in laboratory testing for the viral nucleic acids and antibodies against the virus. The severity of the disease, route of transmission and the lack of prophylaxis fulfils the criteria for the virus being handled as a BSL3/ACDP 3 agent, as are the related viruses SARS-CoV and MERS-CoV (Barkham, 2004). Propagation of SARS-CoV-2 requires the use of BSL3/ACDP3 laboratories (WHO, 2020). In the UK, to provide the extremely high throughput required, the processing of clinical samples for diagnostic purposes has been derogated to BSL2/ACDP2 (PublicHealthEngland, 2020; Bain et al., 2020). To protect laboratory staff conducting SARS-CoV-2 diagnostic testing and research outside of BSL3/ACDP3, effective methods of inactivating live virus present in clinical samples and other virus-infected material are essential.
Heat-treatment at 56 °C for 30 min is commonly used to inactivate complement in serum samples for serological investigations. This has been shown to have no adverse effect on IgA and IgG ELISAs (Hu et al., 2020) and is also used to inactivate live virus present in clinical samples (Huang et al., 2015). This temperature has been found to be effective for many but not all viruses (Park et al., 2016; Pfaender et al., 2015). The effectiveness of heat on the inactivation of SARS-CoV-2 can be influenced by numerous factors including the type of sample, heat source, tube type and the length of time that the samples are heated (e.g. including or excluding the time taken for the samples to reach the target temperature). A recent comparison of available literature for the effect of heat on previously described coronaviruses (Kampf et al., 2020) has shown considerable variation between and within studies. Overall, it concludes that for SARS-CoV and MERS-CoV, heating to 60 °C for 30 min, 65 °C for 15 min and 80 °C for 1 min reduces virus infectivity by at least 4 log10. Previous studies using SARS-CoV have demonstrated that the efficacy of heat inactivation is reduced in samples with higher protein content (e.g. Foetal Calf serum, human serum or Bovine Serum Albumen) (Rabenau et al., 2005; Yunoki et al., 2004; Chang et al., 2020). For successful inactivation the British Standard for virus inactivation (14885:2018 BE, 2018, 14885:2018 BE, 2018) recommends a 4 log 10 or greater reduction in titre.
Similarly, variable results have been reported on the effect of heat on SARS-CoV-2. Complete inactivation of SARS-CoV-2 has been reported at 56 °C after 45 min and 100 °C after 5 min, (Jureka et al., 2020) an increase in titre between 15 and 30 min incubation at 56 °C has also been observed (Jureka et al., 2020). Other studies suggest that SARS-CoV-2 can be inactivated at 56 °C, 65 °C and 95 °C in less than 30 min, 15 min and 3 min respectively. Again, when testing at 37 °C and 42 °C, an increase in titre was observed between 15 and 30 min, and 30 and 60 min respectively (Wang et al., 2020a; Batéjat et al., 2020). For virus-spiked nasopharyngeal and human serum samples, greater than 5 log10 reductions in viral titres have been reported for heat-treatments of 56 °C for 30 min and 60 °C for 60 min. In virus culture supernatant alone, virus titre reductions greater than 6 log10 for 95 °C for 15 min have been demonstrated (Pastorino et al., 2020).
Most of the studies on the effect of heat on coronaviruses have used TCID50 to determine the virus titre in heat-treated compared to untreated samples (Rabenau et al., 2005; Pastorino et al., 2020; Leclercq et al., 2014; Darnell et al., 2004). This may be less sensitive than the use of a plaque assay. None of the studies further subjected the samples to virus culture and RT-PCR to determine the presence of viable virus below the limit of detection of the TCID50 assay.
The aim of this study was to investigate the effect of heat on SARS-CoV-2 viability and subsequent RT-PCR Ct value to provide information to assist decision making for safe inactivation of clinical samples and other virus-infected material prior to diagnostic assays or research conducted at CL2.
2. Materials and methods
2.1. Virus
The virus stock used was a P3 working bank grown from SARS-CoV-2 Strain England 2, a clinical isolate taken during acute illness, propagated in Vero E6 cells. The stock was prepared by infecting 95 % confluent Vero E6 cells with virus to an MOI of 0.005. Virus was harvested after 6 days. The titre was determined to be 7.0 × 105 pfu/mL by plaque assay as described below.
2.2. Heat inactivation
2.2.1. Test samples
For each heat-treatment 3 × 1 mL volumes of virus in tissue culture medium (MEM + 4% FCS) were tested. Heat-treatments of 56 °C, 60 °C and 80 °C for 15, 30 and 60 min, 80 °C for 90 min and 95 °C for 1 and 5 min were tested.
2.2.2. Heat-treatment
Heating was carried out in an unlidded mini dry hot block. The temperature of the different wells in the block was validated before use using a Digitron thermal probe (2024 T) inserted in a non-skirted Sarstedt tube (A2034) containing 1 mL of water. Heating was achieved by placing 1 mL volumes of virus in the hot block at the same time as an identical tube containing 1 mL of water and the thermal probe. The incubation time was started when the liquid in the control tube reached the required temperature (this usually took approximately 10 min). At the end of the incubation time tubes were placed on ice before the plaque assay was carried out. Untreated tubes were left on ice whilst the heating step was carried out.
2.3. Plaque assay
24 well flat-bottomed cell culture plates (Thermo Scientific) were seeded with 3.0 × 105 /mL Vero E6 cells in 0.5 mL volumes of 2 x MEM medium containing Glutamax (2 x MEM Gibco) + 10 % FCS (Sigma,) + 1 x (final concentration) NEAA (Gibco,) + 1 x antibiotic-antimycotic (Gibco) and incubated overnight at 37 °C + 5% CO2. Before use the cells were checked for confluency. Each virus sample was tested in triplicate. Triplicate 10-fold dilutions up to 10−6 of each sample were made in a microtitre plate (Costar). The medium was removed from the 24 well plate and 100 μL of each dilution was added to the appropriate well. Plates were incubated at room temperature with occasional rocking for 1 h, then 0.5 mL overlay (final concentration of 1.5 % CMC (Sigma), 1 x MEM, 4% FCS and 1 x Anti-anti) added to each well. Plates were incubated for 72 h at 37 °C. Plates were fixed for 1 h with 4% formaldehyde in PBS then washed three times with water and allowed to dry. Plates were stained with 250 μL of 1% Crystal Violet (Sigma) for 10–15 min, washed twice with water, dried and the number of plaques counted and recorded.
2.4. Virus detection by serial passage
500 μL of each heat-treated or untreated virus sample was added to a 12.5 cm2 flask of 80 % confluent Vero E6 cells, allowed to adsorb for 60 min at room temperature then 2.5 mL of MEM + 4% FCS was added. Two negative control flasks to which 500 μL MEM + 4% FCS was added in place of virus, were set up in parallel. At the beginning and end of each passage, 140 μl samples of culture supernatant were added to AVL (560 μl; QIAamp viral RNA mini kit (Qiagen) in duplicate. After 10 min, 560 μL of 100 % ethanol was added. Nucleic acids were extracted according to the manufacturer’s instructions and eluted in 60 μL AVE buffer. RT-PCR analysis was conducted as described in Section 2.6. After one week cells were observed for signs of cytopathic effect (cpe) by viewing under a low magnification microscope. Samples for which no cpe was observed were passaged using the above method up to 3 times to allow amplification of any low levels of virus present in the sample. After the first passage a single positive (control) and negative (uninfected) flask were passaged on.
2.5. Heat inactivation and RNA extraction from dilutions of SARS-CoV-2
One ml volumes of SARS-CoV-2 diluted to 7.0 × 104, 7.0 × 102 and 7.0 pfu/mL were heat treated as described in Section 2.2.2 Duplicate 140 μL samples from each tube were taken into 560 μL AVL and RNA extracted as described in Section 2.4.
2.6. RT-PCR
Nucleic acids were stored frozen at -80 °C until they were subjected to RT-PCR in suitable batches with initial (day 0) and final (day 7) samples from each passage in the same run. RT-PCR was carried out on an Applied Biosystems Fast 7500 PCR machine in standard run mode using the SARS-CoV E Sarbeco assay (Corman et al., 2020; Pezzi et al., 2020) using MS2 as an internal extraction control (Rolfe et al., 2007) and aliquots of SARS-CoV-2/England/2/2020 as a positive control. The master mix comprised E- gene F and R primers and TM-P (400 nM, 400 nM and 200 nM final concentration respectively), MS2 primers and TM probe (20 nM, 20 nM and 40 nM final concentration respectively), 4 x TaqMan® Fast Virus 1-Step Master Mix made up with molecular-grade nuclease free water (Ambion) to a final volume of 15 μl. The amount of template material added was 5 μl. Cycling conditions were 55 °C for 10 min, followed by 94 °C for 3 min then 45 cycles of 95 °C for 15 s and 58 °C for 30 s.
3. Results
3.1. Effect of heat on the viability of SARS-CoV-2
The effect of heat on SARS-CoV-2 virus viability as determined by plaque assay is shown in Table 1 . A 4 log10 or greater reduction in titre was observed in all replicates after 56 °C for 30 min, 80 °C for 90 min and 95 °C for 1 and 5 min. Significant variation in heat-treatment efficacy was observed between replicates. At 56 °C the plaque assay titre increased at the 60 min but not the 30 min time point, whereas at 60 °C the plaque assay titre increased at the 30 min time point and for one of six replicates at the 60 min time point. The sensitivity of the plaque assay was 3 pfu/mL.
Table 1.
The effect of heat on the viability of SARS-CoV-2 England 2 virus assessed by plaque assay, observation of cytopathic effect (cpe) and RT-PCR.
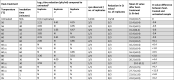 |
*Untreated control virus titre was 5.68 log10 pfu/mL (St dev 0.1; mean 14 replicates); N = no plaques were observed therefore 5.68 log10 titre reduction;2) = duplicate experiment. Shading represents the samples which did not show a 4 log reduction in titre.
In all untreated and 56 °C heated samples, cpe was observed and growth of virus was detected by SARS-CoV specific RT-PCR (as defined by a decrease in Ct value between nucleic acid samples extracted on day 0 and day 7; Table 1). At 60 °C, 3 of 6 replicates (from two separate experiments) showed virus growth (by cpe and RT-PCR) after 15 and 30 min and 5 of 6 after 60 min of heat-treatment. There was greater virus recovery after 60 min incubation at 60 °C compared to shorter incubation times. At 80 °C viable virus was recovered by culture from 3 replicates where no plaques were observed (two after 30 min and one after 60 min heat treatment). No virus was recovered or virus growth detected by RT-PCR from samples heated to 80 °C for 90 min or 95 °C for 1 or 5 min. All replicates showed 100 % correlation between observation of cpe and detection of virus growth by RT-PCR.
3.2. The effect of heat-treatment of virus prior to RNA extraction on RT-PCR assay sensitivity
Heat-treatment of virus to 56 °C or 60 °C had little effect on the sensitivity of RT-PCR. The mean Ct value of 14 untreated replicates was Ct 15.82 (standard deviation. 0.69) compared to the mean Ct value of 9 samples heated to 56 °C or 60 °C of 15.77 (standard deviation 0.55, Table 1). Heating to 80 °C for 30 min or more, however, resulted in an increase of a minimum of three Ct values, equating to a log reduction in sensitivity of the RT-PCR. An increase of a minimum of ten Ct values was observed for samples held at 80 °C for 60 or 90 min. Heating the undiluted virus to 95 °C for 1 or 5 min led to a mean increase in Ct of 6.0 (+/- 0.4) (Table 1, column 8). When virus dilutions were heat-treated to 95 °C for 1 or 5 min there was an increase in Ct value of between 1.6–6.2 (Table 2 ). After 60 min heating at 80 °C the Ct value increased by 10 and 14 for viruses at 7.0 × 104 and 7.0 × 102 pfu/mL respectively, whilst the virus at 7.0 pfu/mL was rendered undetectable by RT-PCR. The effect of heat on Ct value should be considered when interpreting diagnostic PCR results from clinical samples which could have an initial low virus titre.
Table 2.
Ct values of SARS-CoV-2 RNA extracted from heat treated virus.
| Heat-treatment |
Virus concentration |
||||||
|---|---|---|---|---|---|---|---|
| 7.0 × 104 (pfu/mL) |
7.0 × 102 (pfu/mL) |
7.0 (pfu/mL) |
|||||
| Temperature (°C) | Time minutes) | Ct Value |
|||||
| (± St dev) | Difference from unheated | (±St dev) | Difference from unheated | (±St dev) | Difference from unheated | ||
| Untreated | 0 | 17.2 (±0.1) | 0 | 23.2 (±0.1) | 0 | 30.2 (±0.3) | 0 |
| 56 | 60 | 18.6 (±0.1) | +1.4 | 24.3 (±0.1) | +1.1 | 30.5 (±0.2) | +0.3 |
| 80 | 15 | 18.1 (±0.1) | +0.9 | 24.8 (±0.1) | +1.6 | 28.8 (±4.9) | −1.4 |
| 80 | 30 | 21.0 (±0.1) | +3.8 | 28.5 (±0.4) | +5.3 | 34.7 | +4.5 |
| 80 | 60 | 28.1 (±0.5) | +10.9 | 37.5* | +14.3 | U | N/A |
| 80 | 90 | 25.8 (±0.3) | +8.6 | 35.4 (±0.4) | +12.2 | U | N/A |
| 95 | 1 | 18.8 (±0.3) | +1.6 | 26.6 (±0.8) | +3.4 | 32.5 (±0.4) | +2.3 |
| 95 | 5 | 21.7 (±0.2) | +4.5 | 29.4 (±0.6) | +6.2 | 36.2 | +6 |
Each Ct value is the mean from duplicate RNA extractions.
Only one sample gave a Ct value. U = Ct undetermined (RNA not detected); N/A = Not applicable.
4. Discussion
Determination of the effect of heat-treatment on the virus titre of SARS-CoV-2 is an important consideration when using heat to inactivate SARS-CoV-2 prior to conducting diagnostic assays at lower containment levels. It is important to develop a method of safely inactivating virus whilst maintaining the sensitivity of the subsequent diagnostic assay. Several studies on the effect of heat on closely related MERS, SARS and SARS CoV-2 have generated varying results. In this study a 4 log10 or greater reduction in virus titre was consistently observed in all replicates after 56 °C for 30 min. As independent confirmation of our results, heat-treatment at 56 °C was also tested in a different PHE laboratory by TCID50 using a separate virus stock (concentration 7.0 log10 TCID50/mL). After 15, 30 and 60 min a 3.5, 5.3 and 4.3 log10 TCID50/mL reduction in titre was observed. This is also in agreement with results described by Pastorino et al. (2020). It contradicts the findings of other studies where 56 °C for 30 min (Wang et al., 2020a; Batéjat et al., 2020); or 45 min (Jureka et al., 2020) or 80 °C for 60 min (Patterson et al., 2020) was shown to completely inactivate the virus. In this study complete inactivation was only observed after heating to 80 °C for 90 min and 95 °C for 1 and 5 min. Successful heat inactivation after 5 min heating at 95 °C has also been recently demonstrated by plaque assay (Smyrlaki et al., 2020). The considerable variation between studies could be due to the method of virus titration. This study used plaque assays (the gold standard for quantifying replication‐competent lytic virions as plaque-forming units (Mendoza et al., 2020), serial passage of virus in cell culture and RT-PCR in combination to demonstrate the presence of viable virus below the limit of detection of the plaque assay. These methodologies in combination are more sensitive than using titration alone. Continuation of virus culture for three serial passages was conducted to determine whether low levels of virus were present in the samples which became apparent after further passage. In this study no virus was observed in after the first passage.
Variation could also be potentially due to the volumes of virus heated. In this study 1 mL volumes were tested to allow triplicate plaque assays and virus culture to be carried out. For high-throughput processing a significantly lower amount is usually required depending on the method and/or platform used. The other studies quoted tested volumes of 500 μL or less. In this study it took approximately 10 min for the tube contents to reach temperature in the hot block. This was affected by the number of tubes as well as the volume of liquid being heated. This highlights the importance of using an independent temperature-monitoring tube as a control during each heat-treatment and testing labs should consider the time taken for the tube contents to reach temperature in the hot block in their inactivation procedures. Another consideration is the potential variation in temperature that can occur when using a hot block. The hot block in this study was carefully monitored (the temperature in all the positions of the hot block were mapped), however there have been reports that the use of hot blocks can result in unequal heating, hotspots, or spikes in temperature (Nguyen et al., 2015). Many diagnostic laboratories use water baths rather than hot blocks, which may differ in terms of susceptibility to temperature variation.
Interestingly, in this study at both 56 °C and 60 °C the number of plaques observed increased with longer treatment times. This is in agreement with findings using SARS-CoV (Darnell et al., 2004) and has also been reported for SARS-CoV-2 at 56 °C (Jureka et al., 2020) and at 37 °C and 42 °C (Wang et al., 2020a). One possible explanation for this may be the formation and subsequent dissociation of virus aggregates in response to heat (Darnell et al., 2004). Virus aggregates would produce individual plaque identical to those observed for individual virions, resulting in an underestimate of the true number of infectious virus particles in aggregated samples. True inactivation of disaggregated virions may occur after longer incubation times or at higher temperatures. Additional work would be required to investigate this further.
Real-time RT-PCR cycle threshold (Ct) values are defined as the number of cycles of amplification required for the accumulated fluorescence (produced by target gene amplification) to reach a threshold above the background. Ct values are therefore inversely related to viral load; low Ct values indicate high viral loads and high Ct values indicate low virus nucleic acid concentration in the sample. With regards to SARS-CoV-2, low Ct values from patient samples have been reported to correlate with increased probability of progression to severe disease and mortality (Rao et al., 2020). In this study the Ct value was not significantly affected by heating to 56 °C and 60 °C. This is in agreement with some studies (Pastorino et al., 2020; Wang et al., 2020b) but not others (Pan et al., 2020; Zou et al., 2020). We also found that heating to 80 °C for 30 min or more led to an increase in Ct value and therefore a reduction in RT-PCR sensitivity that could impact upon clinical diagnosis. The less notable increase in Ct value observed when virus was heated to 95 °C in this study could be attributed to the shorter heating time. Further work needs to be done before in-depth conclusions can be drawn, however this should be a consideration for downstream processing requiring high sensitivity such as clinical diagnostic RT-PCRs. Heat-treatment at lower temperatures combined with chemical inactivation, short duration high-temperature heat-treatments, or chemical inactivation alone may be more appropriate to preserve RNA integrity and optimise PCR detection of SARS-CoV-2 RNA from low titre clinical samples.
In this study the effect of heat was tested on virus-infected tissue culture supernatant. It is likely that in some cases heat-treatment would be even more variable in clinical samples, although this has not always been reported (Wang et al., 2004). Our results show significant variation in the effectiveness of heat-treatment for inactivation of SARS-CoV-2. This emphasises the importance of local validation of inactivation methods and the need for consistency in inactivation protocols to ensure sufficient reduction in virus titre for processing of clinical samples and research material in BSL2/ACDP2 facilities.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author statement
Conception and design of study: Jane Burton: Marian Killip; Kevin Richards; Christine Bruce; Allen D G Roberts.
Acquisition of data: Jane Burton; Hannah Love; Kevin Richards; Christopher Burton; Sian Summers; James Pitman; Linda Easterbrook; Katherine Davies
Analysis and/or interpretation of data: Jane Burton; Hannah Love; Peter Spencer; Marian Killip;
Drafting the manuscript: Jane Burton; Hannah Love
Revising the manuscript critically for important intellectual content: Katherine Davies; Marian Killip; Patricia Cane; Christine Bruce
Approval of the version of the manuscript to be published (the names of all authors must be listed): Jane Burton, Hannah Love, Kevin Richards, Christopher Burton, Sian Summers, James Pitman, Linda Easterbrook, Katherine Davies, Peter Spencer, Marian Killip, Patricia Cane, Christine Bruce, Allen D G Roberts.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
None.
References
- 14885:2018 BE . Application of European Standards for Chemical Disinfectants and Antiseptics. 2018. Chemical disinfectants and antiseptics. [Google Scholar]
- Bain W., Lee J.S., Watson A.M., Stitt-Fischer M.S. Practical guidelines for collection, manipulation and inactivation of SARS-CoV-2 and COVID-19 clinical specimens. Curr. Protoc. Cytom. 2020;93:e77. doi: 10.1002/cpcy.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkham T.M. Laboratory safety aspects of SARS at Biosafety Level 2. Ann. Acad. Med. Singapore. 2004;33:252–256. [PubMed] [Google Scholar]
- Batéjat C., Grassin Q., Manuguerra J.-C., Leclercq I. 2020. Heat Inactivation of the Severe Acute Respiratory Syndrome Coronavirus 2. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Yan Y., Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfus. Med. Rev. 2020;34(Apr(2):75–80. doi: 10.1016/j.tmrv.2020.02.003. Epub 2020 Feb 21. PMID: 32107119; PMCID: PMC7135848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020:25. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell M.E., Subbarao K., Feinstone S.M., Taylor D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol. Methods. 2004;121:85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Zhang R., An T., Li Q., Situ B., Ou Z., Wu C., Yang B., Tian P., Hu Y., Ping B., Wang Q., Zheng L. Impact of heat-inactivation on the detection of SARS-CoV-2 IgM and IgG antibody by ELISA. Clin. Chim. Acta. 2020;509(Oct):288–292. doi: 10.1016/j.cca.2020.06.032. Epub 2020 Jun 20. PMID: 32569631; PMC7305743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.J., Hsu W.W., Higgs S., Vanlandingham D.L. Temperature tolerance and inactivation of Chikungunya virus. Vector Borne Zoonotic Dis. 2015;15:674–677. doi: 10.1089/vbz.2015.1795. [DOI] [PubMed] [Google Scholar]
- Jureka A.S., Silvas J.A., Basler C.F. Propagation, inactivation, and safety testing of SARS-CoV-2. Viruses. 2020:12. doi: 10.3390/v12060622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Voss A., Scheithauer S. Inactivation of coronaviruses by heat. J. Hosp. Infect. 2020;105(Jun(2):348–349. doi: 10.1016/j.jhin.2020.03.025. Epub 2020 Mar 31. PMID: 32243951; PMCID: PMC7271332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq I., Batéjat C., Burguière A.M., Manuguerra J.C. Heat inactivation of the Middle East respiratory syndrome coronavirus. Influenza Other Respir. Viruses. 2014;8:585–586. doi: 10.1111/irv.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza E., Manguiat K., Wood H., Drebot M. Two detailed plaque assay protocols for the quantification of infectious SARS‐CoV‐2. Curr. Protoc. Microbiol. 2020:57. doi: 10.1002/cpmc.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.B., Clifford V., Wahab A.A., Sinickas V. Heat treatment of biochemical samples to inactivate Ebola virus: does it work in practice? Hong Kong Med. J. 2015;21:378. doi: 10.12809/hkmj154629. [DOI] [PubMed] [Google Scholar]
- Pan Y., Long L., Zhang D., Yan T., Cui S., Yang P., Wang Q., Ren S. Potential false-negative nucleic acid testing results for severe scute respiratory syndrome coronavirus 2 from thermal inactivation of samples with low viral loads. Clin. Chem. 2020;66(Jun 1(6):794–801. doi: 10.1093/clinchem/hvaa091. PMID: 32246822; PMCID: PMC7184485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.L., Huang Y.J., Hsu W.W., Hettenbach S.M., Higgs S., Vanlandingham D.L. Virus-specific thermostability and heat inactivation profiles of alphaviruses. J. Virol. Methods. 2016;234:152–155. doi: 10.1016/j.jviromet.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Pastorino B.T.F., Gilles M., de Lamballerie X., Charrel R.N. Heat inactivation of different types of SARS-CoV-2 samples: what protocols for biosafety, molecular detection and serological diagnostics? Viruses. 2020;12:735. doi: 10.3390/v12070735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson E.I.P.T., Anderson E.R., Casas-Sanchez A., Smith S.L., Cansado-Utrilla C., Turtle L., Hughes G.L. Methods of inactivation of SARS-CoV-2 for downstream biological assays. Prepr Serv Biol. 2020 doi: 10.1093/infdis/jiaa507. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzi L., Charrel R.N., Ninove L., Nougairede A., Molle G., Coutard B., Durand G., Leparc-Goffart I., de Lamballerie X., Thirion L. Development and evaluation of a duo SARS-CoV-2 RT-qPCR assay combining two assays approved by the world health organization targeting the envelope and the RNA-Dependant RNA polymerase (RdRp) coding regions. Viruses. 2020:12. doi: 10.3390/v12060686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaender S., Brinkmann J., Todt D., Riebesehl N., Steinmann J., Steinmann J., Pietschmann T., Steinmann E. Mechanisms of methods for hepatitis C virus inactivation. Appl. Environ. Microbiol. 2015;81:1616–1621. doi: 10.1128/AEM.03580-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PublicHealthEngland . 2020. COVID-19: Guidance for Sampling and for Diagnostic Laboratories. [Google Scholar]
- Rabenau H.F., Cinatl J., Morgenstern B., Bauer G., Preiser W., Doerr H.W. Stability and inactivation of SARS coronavirus. Med. Microbiol. Immunol. 2005;194:1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.N., Manissero D., Steele V.R., Pareja J. A systematic review of the clinical utility of cycle threshold values in the context of COVID-19. Infect. Dis. Ther. 2020;9:573–586. doi: 10.1007/s40121-020-00324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe K.J., Parmar S., Mururi D., Wreghitt T.G., Jalal H., Zhang H., Curran M.D. An internally controlled, one-step, real-time RT-PCR assay for norovirus detection and genogrouping. J. Clin. Virol. 2007;39:318–321. doi: 10.1016/j.jcv.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Smyrlaki I., Ekman M., Lentini A., Rufino de Sousa N., Papanicoloau N., Vondracek M., Aarum J., Safari H., Muradrasoli S., Gigliotti Rothfuchs A., Albert J., Högberg B., Reinius B. 2020. Massive and Rapid COVID-19 Testing Is Feasible by Extraction-free SARS-CoV-2 RT-PCR. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wu X., Wang Y., Li B., Zhou H., Yuan G., Fu Y., Luo Y. Low stability of nucleocapsid protein in SARS virus. Biochemistry. 2004;43:11103–11108. doi: 10.1021/bi049194b. [DOI] [PubMed] [Google Scholar]
- Wang T., Lien C., Liu S., Selveraj P. 2020. Effective Heat Inactivation of SARS-CoV-2. medRxiv. [DOI] [Google Scholar]
- Wang Y., Song W., Zhao Z., Chen P., Liu J., Li C. The impacts of viral inactivating methods on quantitative RT-PCR for COVID-19. Virus Res. 2020;285 doi: 10.1016/j.virusres.2020.197988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. World Health Organization Laboratory Biosafety Guidance Related to the Novel Coronavirus (2019-nCoV) [Google Scholar]
- Yunoki M., Urayama T., Yamamoto I., Abe S., Ikuta K. Heat sensitivity of a SARS-associated coronavirus introduced into plasma products. Vox Sang. 2004;87:302–303. doi: 10.1111/j.1423-0410.2004.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J., Zhi S., Chen M., Su X., Kang L., Li C., Su X., Zhang S., Ge S., Li W. Heat inactivation decreases the qualitative real-time RT-PCR detection rates of clinical samples with high cycle threshold values in COVID-19. Diagn. Microbiol. Infect. Dis. 2020;98 doi: 10.1016/j.diagmicrobio.2020.115109. 115109-115109. [DOI] [PMC free article] [PubMed] [Google Scholar]


