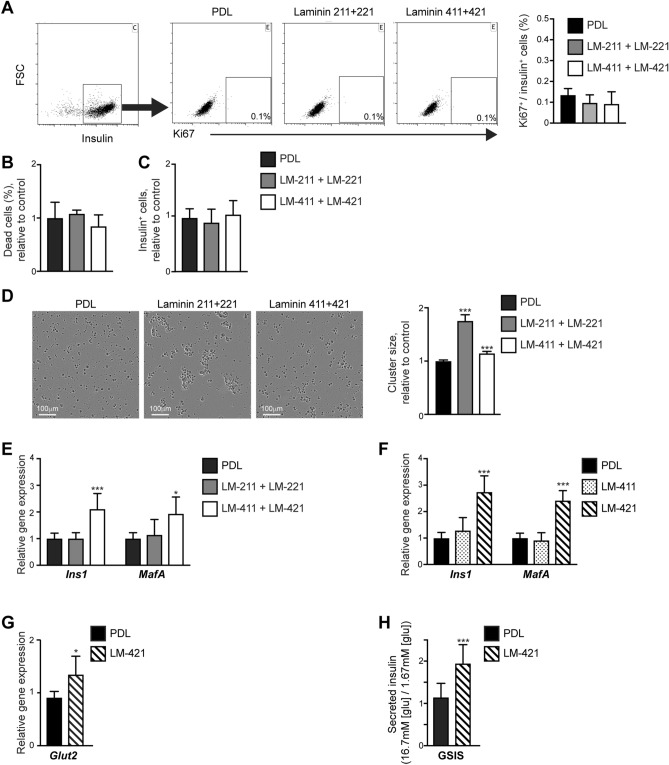
Figure 3.
Pericytic laminins influence islet cells clustering, gene expression, and function. Dispersed mouse islet cells were cultured on either Poly-D-Lysine (PDL) or indicated recombinant laminin combinations (LM): LM-211 and -221 mixture (gray bars); LM-411 and -421 mixture (empty bars); LM-411 (dotted bars); or LM-421 (striped bars). (A) After cultured for 48 h, cells were stained for insulin and the proliferating cell marker Ki67 and analyzed by flow cytometry. Left, a representative dot plot of insulin staining. Middle, representative dot plots of Ki67 labeling of insulin+ cells under each culture condition. Right, a bar diagram (mean ± SD) shows the percentage of Ki67+ cells of insulin+ cells. n = 4. (B) Bar diagram (mean ± SD) shows the relative percentage of dead cells upon culturing on PDL (the average was set to '1’) or laminins. Dead cells were identified as DAPI+ unfixed cells. n = 3–4. C, Bar diagram (mean ± SD) shows the relative number of insulin-expressing cells upon culturing on PDL (the average was set to ‘1’) and laminins. n = 5. (D) Clustering of dispersed islet cells for 72 h. Left, representative images. Right, bar diagram (± SEM) shows the relative cluster size. n > 600 clusters for each condition. (E–G) Bar diagram shows a qPCR analysis of indicated genes upon culturing for 72 h. The averages of PDL-cultured cells were set to '1’. n = 4–5. (H) Bar diagram shows glucose-stimulated insulin secretion (GSIS) analysis. After a 72-h culture on either PDL or LM-421, cells were incubated with low (1.67 mM) glucose concentration, followed by incubation with high (16.7 mM) glucose concentration. The levels of secreted insulin were measured, and the ratio between levels secreted in response to high and low glucose levels was determined for each well. n = 6–7. *, p < 0.05; ***, p < 0.005, as compared to PDL-treated cells.