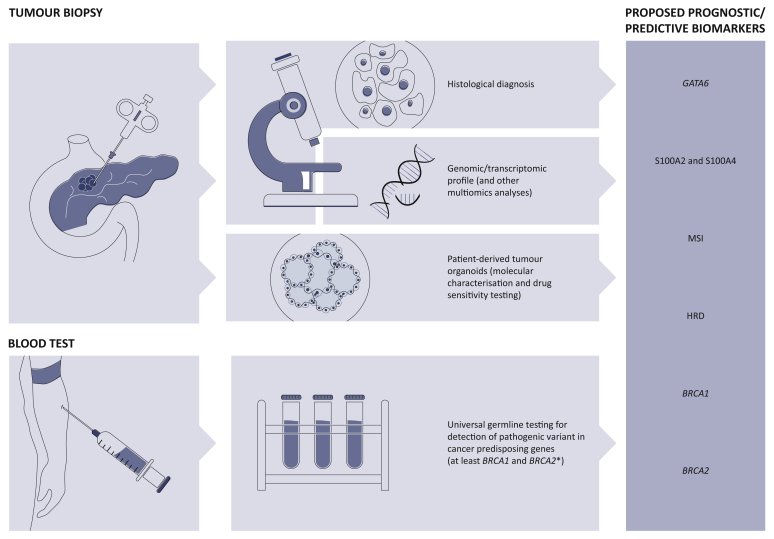
Figure 4.
Proposed biomarkers to be implemented in future neoadjuvant clinical trials.
HRD: homologous recombination deficiency, defined by germline or somatic mutations in HRD-related genes (BRCA1/2, ATM, PALB2, FANC-genes, RAD51, etc.), COSMIC3 signature, or genomic instability through structural variation patterns; MSI: microsatellite instability.
∗NCCN guidelines have been recently updated and recommend universal screening for germline variant in patients with PC, regardless of age, ethnicity, and family/personal history of cancer, including not only BRCA1/2 but also ATM, CDKN2A, PALB2, STK11, TP53, MLH1, MSH2, MSH6, and PMS2.31