Abstract
Recent studies suggest that microRNAs (miRNAs) are critical regulators in many types of cancer, including osteosarcoma. miR-342-3p has emerged as an important cancer-related miRNA in several types of cancers. However, the functional significance of miR-342-3p in osteosarcoma is unknown. The aims of this study were to investigate whether miR-342-3p is dysregulated in osteosarcoma and to explore the biological function of miR-342-3p in regulating cellular processes of osteosarcoma cells. We found that miR-342-3p expression was significantly decreased in osteosarcoma tissues and cell lines. Overexpression of miR-342-3p inhibits the proliferation, migration, and invasion of osteosarcoma cells. In contrast, the inhibition of miR-342-3p exhibited the opposite effect. Astrocyte-elevated gene-1 (AEG-1) was identified as one of the target genes of miR-342-3p in osteosarcoma cells by bioinformatics analysis, dual-luciferase reporter assay, real-time quantitative polymerase chain reaction, and Western blot analysis. Overexpression of miR-342-3p also inhibited the Wnt and nuclear factor κB signaling pathways. Moreover, overexpression of AEG-1 partially rescued the inhibitory effects of miR-342-3p mediated on the proliferation, migration, and invasion of osteosarcoma cells. Overall, our results show that miR-342-3p inhibits the proliferation, migration, and invasion of osteosarcoma cells through targeting AEG-1, suggesting a potential target for the development of miRNA-based therapy for osteosarcoma.
Key words: Astrocyte-elevated gene-1 (AEG-1), miR-342-3p, Osteosarcoma, Wnt, Nuclear factor κB (NF-κB)
INTRODUCTION
Osteosarcoma is the most common malignant bone tumor with a high morbidity in children and adolescents1,2. Osteosarcoma is a refractory disease because of its high local aggressiveness and its potential for rapid metastasis3. Despite the advances in treatment strategies over the past few decades, the prognosis and survival rate of osteosarcoma are still poor3. The pathogenesis of osteosarcoma is related to aberrant genetic and epigenetic alterations that lead to dysregulation of oncogenes or tumor suppressor genes4. However, a good understanding of the pathogenesis of osteosarcoma remains a challenge. Therefore, it is essential to gain a better understanding of the underlying mechanism of osteosarcoma to help with the development of novel strategies for the diagnosis, prognosis, and treatment of osteosarcoma patients.
MicroRNAs (miRNAs) are a group of short, noncoding RNAs, usually 18–25 nucleotides in length, that negatively regulate gene expression through base pairing to the 3′-untranslated region (3′-UTR) of the target genes5,6. It has been documented that miRNAs play a critical role in various activities, such as development, cellular differentiation programs, and oncogenesis7. In particular, miRNAs modulate various cellular processes in cancer, including proliferation, apoptosis, migration, and invasion8,9. The dysregulation of miRNA expression has been suggested to be an important mechanism for tumorigenesis10,11. Thus, miRNAs have a therapeutic potential in the treatment of cancer12,13. Increasing evidence has also reported that various miRNAs are dysregulated in osteosarcoma and participate in the development and progression of osteosarcoma14–17. Thus, miRNAs have emerged as potential and promising targets for the prognosis, diagnosis, and treatment of osteosarcoma. However, the precise role of miRNAs in osteosarcoma remains largely unknown.
Astrocyte-elevated gene-1 (AEG-1), also known as metadherin, has been suggested as an oncogene in recent years18–21. AEG-1 is originally induced in human fetal astrocytes and characterized as a human immunodeficiency virus-1- and tumor necrosis factor-α-inducible gene22. AEG-1 is found to be highly expressed in numerous cancers, including lung cancer23, hepatocellular carcinoma24, and glioma25. AEG-1 is associated with various oncogenic signaling pathways such as Wnt and nuclear factor κB (NF-κB), which regulate cancer growth and metastasis26. AEG-1 also plays an important role in osteosarcoma. AEG-1 is highly expressed in osteosarcoma tissues and is positively correlated with clinical stage, metastasis, and poor survival of osteosarcoma patients27,28. AEG-1 promotes osteosarcoma cell proliferation, metastasis, and chemoresistance associated with activation of the Wnt, endothelin, Jun N-terminal kinase, and NF-κB signaling pathways29–31. Therefore, AEG-1 may serve as a potential and promising therapeutic target for osteosarcoma treatment.
miR-342-3p has been reported to be an important cancer-related miRNA in several types of cancers32,33. However, the functional significance of miR-342-3p in osteosarcoma is unknown. In this study, we showed that miR-342-3p expression was significantly decreased in osteosarcoma tissues and cell lines. Overexpression of miR-342-3p inhibits the proliferation, migration, and invasion of osteosarcoma cells. AEG-1 was identified as one of the target genes of miR-342-3p in osteosarcoma cells. miR-342-3p also regulates the Wnt and NF-κB signaling pathways by targeting AEG-1. Overall, our results show that miR-342-3p inhibits the proliferation, migration, and invasion of osteosarcoma cells through the downregulation of AEG-1, suggesting a potential therapeutic target for the development of miRNA-based therapy for osteosarcoma treatment.
MATERIALS AND METHODS
Tissue Specimen Selection
Paired osteosarcoma tissue samples and adjacent nontumor tissues were obtained from 20 patients undergoing surgery at The First Hospital of Jilin University. The resected tissues were immediately snap frozen in liquid nitrogen and stored at −80°C before use. All patients agreed and signed the written informed consent prior to sample collection. This study was reviewed and approved by the Human Research and Ethics Committee of The First Hospital of Jilin University and conducted in accordance with the Declaration of Helsinki.
Cell Lines and Cell Culture
The normal human osteoblast cell line hFOB1.19 and human osteosarcoma cell lines (MG63, U2OS, SOSP-9607, and HOS) were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, P.R. China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Rockville, MD, USA) containing 10% fetal bovine serum (FBS), streptomycin (100 μg/ml), and penicillin (100 U/ml). All cells were incubated at 37°C in a humidified incubator of 5% CO2.
Cell Transfection
The miR-342-3p mimics, miR-342-3p inhibitor, and scramble negative control (NC) were synthesized by GenePharma (Shanghai, P.R. China). The AEG-1 cDNA without 3′-UTR was cloned into pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA) to generate the pcDNA3.1-AEG-1 vector. Cell transfection was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol.
RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted using TRIzol (Invitrogen) and miRNeasy mini kit (Qiagen, Dusseldorf, Germany) according to the manufacturers’ protocols. RNA was converted to cDNA using Moloney murine leukemia virus reverse transcriptase (Takara, Dalian, P.R. China) and TaqMan MicroRNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). PCR was performed using a SYBR Green PCR kit (Applied Biosystems) on a 7900HT system (Applied Biosystems). The PCR conditions were as follows: 95°C for 5 min; 35 cycles at 95°C for 5 s; 55°C for 30 s; 72°C for 30 s; and 72°C for 5 min. GAPDH and U6 were used as internal controls. Relative gene expression was calculated by the 2−ΔΔCt method. Fold changes of gene expression were obtained by normalization with the control group. The primers used were as follows: miR-342-3p, 5′-TGCGGTCTCACACAGAAATCGCAC-3′ (forward) and 5′-CCAGTGCAGGGTCCGAGGT-3′ (reverse); U6, 5′-CTCGCTTCGGCAGCACA-3′ (forward) and 5′-AACGCTTCACGAATTTGCGT-3′ (reverse); AEG-1, 5′-CGAGAAGCCCAAACCAAATG-3′ (forward) and 5′-TGGTGGCTGCTTTGCTGTT-3′ (reverse); cyclin D1, 5′-CCGTCCATGCGGAAGATC-3′ (forward) and 5′-GAAGACCTCCTCCTCGCACT-3′ (reverse); matrix metalloproteinase-2 (MMP-2), 5′-AGGCCAAGTGGTCCGTGTGA-3′ (forward) and 5′-TAGGTGGTGGAGCACCAGAG-3′ (reverse); GAPDH, 5′-GACTCATGACCACAGTCCATGC-3′ (forward) and 5′-AGAGGCAGGGATGATGTTCTG-3′ (reverse).
Cell Proliferation Assay
Cell proliferation was detected by the cell counting kit-8 (CCK-8; Sigma-Aldrich, St. Louis, MO, USA). Briefly, cells were seeded into 96-well plates at a concentration of 5,000 cells per well and cultured for 48 h after transfection. Cells were then incubated in 10% CCK-8 reagent at 37°C. Absorbance at 450 nm was measured using a microplate reader (Bio-Tek Instruments, Winooski, VT, USA).
Cell Cycle Assay
Cells were serum starved for 24 h to synchronize the cell cycle. After transfection for 48 h, cells were digested with trypsin, washed with phosphate-buffered saline (PBS), and then fixed with 70% ethanol. Cells were incubated with 100 μg/ml of propidium iodide (Sigma-Aldrich) and 10 μg/ml of RNase A for 30 min in the dark. Cell cycle distribution was detected by a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA), and data were analyzed by CellQuest software.
Cell Migration and Invasion Assays
Cells transfected with miR-342-3p mimic or miR-342-3p inhibitor were harvested 48 h after transfection and subjected to cell invasion and migration assays. Cells in serum-free DMEM were seeded into the top chamber of the Transwell chamber, whereas DMEM containing 10% FBS was added to the bottom chamber. Cells were cultured for 24 h at 37°C. Cells remaining on the top side of the membrane were removed, and cells in the bottom chamber were stained with 0.1% crystal violet (Sigma-Aldrich). Cells were counted at 10× magnification under a microscope (Olympus, Tokyo, Japan). For the invasion assay, Transwell inserts were precoated with Matrigel matrix (BD Biosciences), and the assay was performed in the same manner as the migration assay.
Dual-Luciferase Reporter Assay
To detect the targeting relationship between miR-342-3p and AEG-1 3′-UTR, the AEG-1 3′-UTR containing the seed-matched sequences or mutated sequences of miR-342-3p was cloned into pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega, Madison, WI, USA). The miR-342-3p mimic or miR-342-3p inhibitor and pmirGLO-AEG-1 (WT) or mirGLO-AEG-1 (MT) were cotransfected into the MG63 cells using Lipofectamine 2000. To detect Wnt activity, cells were cotransfected with miR-342-3p mimic or miR-342-3p inhibitor and TOPFlash firefly luciferase reporter vector along with phRL-TK Renilla luciferase vectors (Promega). To detect NF-κB activity, cells were cotransfected with miR-342-3p mimic or miR-342-3p inhibitor and pNF-κB-luciferase vector (Promega) along with phRL-TK Renilla luciferase vectors (Promega). Relative luciferase activity was detected 48 h after transfection using a dual-luciferase assay kit (Promega).
Western Blot
Cells were lysed, and protein concentrations were measured using a bicinchoninic acid kit (Beyotime, Haimen, P.R. China). Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Millipore, Boston, MA, USA). After blocking with 5% nonfat milk for 1 h, the membrane was immunoblotted with primary antibodies (anti-AEG-1 and anti-GAPDH; Abcam, Cambridge, UK) overnight at 4°C followed by horseradish peroxidase-linked secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The signal was detected using an enhanced chemiluminescence system (Millipore). Gray values of protein bands were measured by Image-Pro Plus 6.0 software.
Data Analysis
Data were reported as the mean ± standard deviation. The difference between groups was analyzed using Student’s t-test or one-way ANOVA followed by the Bonferroni test. Statistical analyses were performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). A value of p < 0.05 was regarded as statistically significant.
RESULTS
miR-342-3p Is Decreased in Osteosarcoma Tissues and Cell Lines
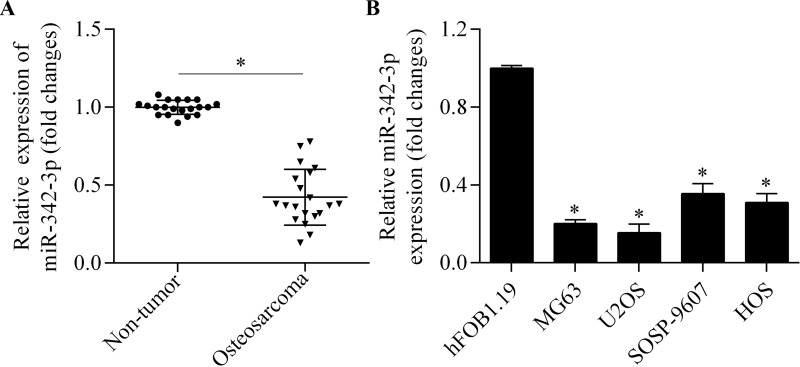
To determine the potential relevance of miR-342-3p in osteosarcoma, we first examined the expression changes in osteosarcoma tissues by RT-qPCR. miR-342-3p was significantly decreased in osteosarcoma tissues compared with adjacent nontumor tissues (Fig. 1A). We further detected the miR-342-3p expression in four osteosarcoma cell lines and in a normal human osteoblast cell line. We found that the expression levels of miR-342-3p in osteosarcoma cell lines were much lower than in normal cell lines (Fig. 1B). This indicates a suppressed expression of miR-342-3p in osteosarcoma.
Figure 1.
Downregulation of miR-342-3p in osteosarcoma. (A) Real-time quantitative polymerase chain reaction (RT-qPCR) analysis of miR-342-3p expression in osteosarcoma tissues and adjacent nontumor tissues. *p < 0.05. (B) RT-qPCR analysis of miR-342-3p expression in four osteosarcoma cell lines (MG63, U2OS, SOSP-9607, and HOS) and normal human osteoblast cell line hFOB1.19. *p < 0.05 versus hFOB1.19.
miR-342-3p Inhibits Proliferation of Osteosarcoma Cells
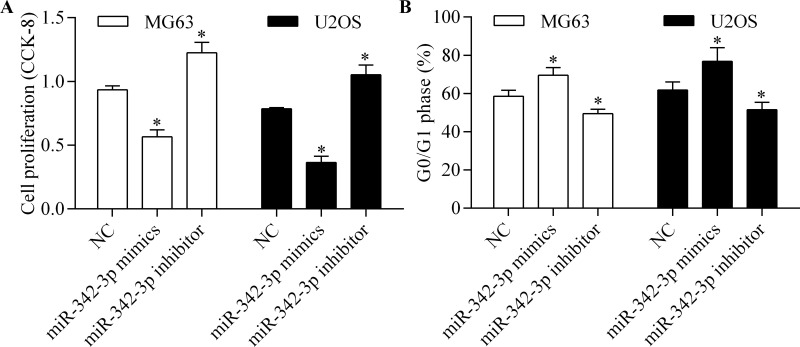
To investigate the biological function of miR-342-3p, we performed gain-of-function and loss-of-function experiments by transfection of miR-342-3p mimic or miR-342-3p inhibitor into MG63 and U2OS cells. We then detected their effects on osteosarcoma cell proliferation using CCK-8. The results showed that miR-342-3p overexpression significantly inhibited osteosarcoma cell proliferation, whereas miR-324-3p inhibition markedly promoted the proliferation of osteosarcoma cells (Fig. 2A). Furthermore, we detected the role of miR-342-3p on cell cycle progression of osteosarcoma cells by flow cytometry. miR-342-3p overexpression induced an increase in G0/G1 phase arrest, whereas miR-342-3p inhibition showed the opposite effect (Fig. 2B). This suggests that miR-342-3p inhibits the proliferation of osteosarcoma cells.
Figure 2.
miR-342-3p inhibits osteosarcoma cell proliferation. MG63 and U2OS cells were transfected with miR-342-3p mimics or miR-342-3p inhibitor for 48 h. (A) Cell proliferation was assessed by cell counting kit-8 (CCK-8). (B) Cell cycle distribution was detected by flow cytometry. *p < 0.05 versus NC.
miR-342-3p Inhibits Migration and Invasion of Osteosarcoma Cells
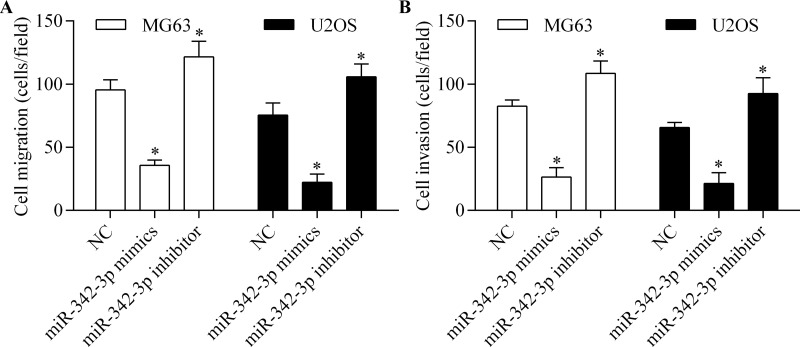
To further investigate the antitumor effect of miR-342-3p on osteosarcoma cells, we analyzed the role of miR-342-3p in regulating osteosarcoma cell migration and invasion. The results showed that both migration (Fig. 3A) and invasion (Fig. 3B) of osteosarcoma cells were significantly reduced by miR-342-3p overexpression. However, miR-342-3p inhibition markedly increased the migration (Fig. 3A) and invasion (Fig. 3B) of osteosarcoma cells. Overall, these results suggest that miR-342-3p impedes the migration and invasion of osteosarcoma cells.
Figure 3.
miR-342-3p impedes the migration and invasion of osteosarcoma cells. Cell migration (A) and invasion (B) of MG63 and U2OS cells were detected by Transwell assay. Cells were transfected with miR-342-3p mimic or miR-342-3p inhibitor for 48 h and then subjected to Transwell assay for 24 h. *p < 0.05 versus NC.
miR-342-3p Targets the 3′-UTR of AEG-1 and Regulates AEG-1 Expression
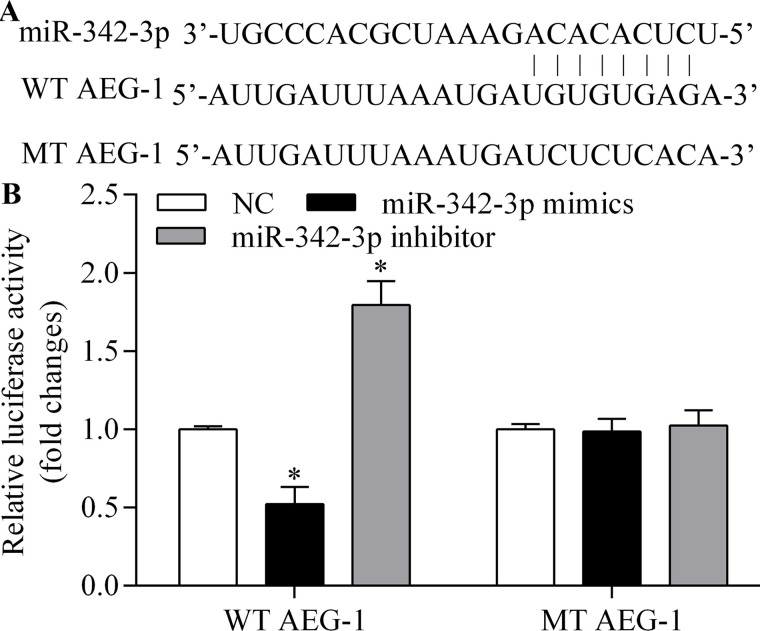
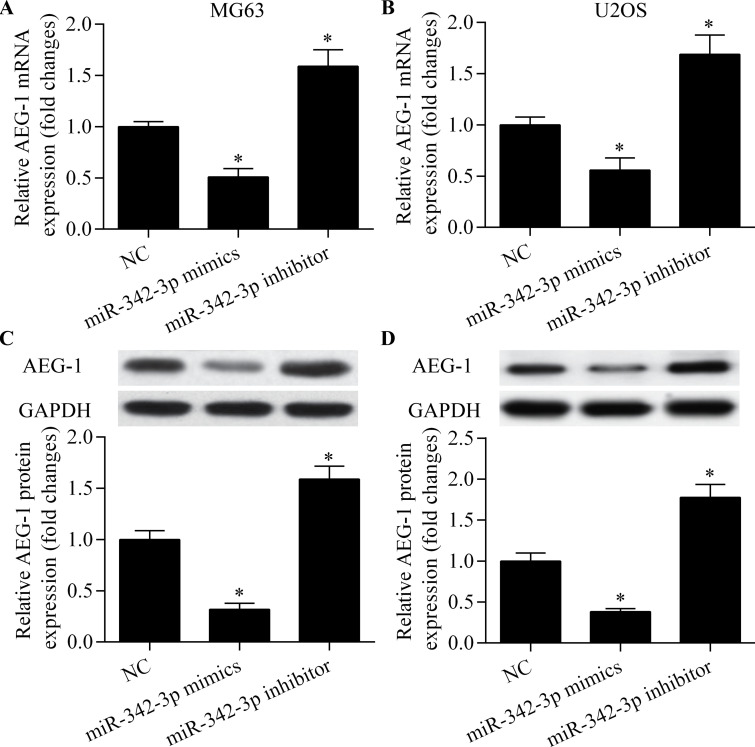
To identify the functional target of miR-342-3p in osteosarcoma cells, we predicted the targets of miR-342-3p using bioinformatics analysis. AEG-1 was among the predicted target genes of miR-342-3p. AEG-1 is highly expressed in osteosarcoma and relates to tumorigenesis, metastasis, and chemoresistance of osteosarcoma cells27,29,30. The putative binding sites are shown in Figure 4A. To test whether AEG-1 is a direct target gene of miR-342-3p, we cloned AEG-1 3′-UTR and its corresponding mutant counterparts into a pmirGLO vector. These vectors were then cotransfected into MG63 cells with miR-342-3p mimic or miR-342-3p inhibitor. We found that miR-342-3p overexpression significantly reduced the luciferase activity in cells transfected with the wild-type (WT) 3′-UTR of AEG-1 but not in cells with mutant (MT) 3′-UTR (Fig. 4B). In contrast, miR-342-3p inhibition significantly increased the luciferase activity in cells transfected with the WT 3′-UTR of AEG-1 but not in cells with MT 3′-UTR (Fig. 4B). To test whether AEG-1 expression is regulated by miR-342-3p, we detected the effect of miR-342-3p on AEG-1 expression. We found that miR-342-3p overexpression led to the inhibition of AEG-1 mRNA and protein expression (Fig. 5A–D). In contrast, miR-342-3p inhibition significantly promoted AEG-1 expression (Fig. 5A–D). Taken together, these results indicate that miR-342-3p targets the 3′-UTR of AEG-1 and regulates AEG-1 expression.
Figure 4.
miR-342-3p targets the 3′-untranslated region (3′-UTR) of AEG-1. (A) Schematic diagram of miR-342-3p predicted binding sites for AEG-1 3′-UTR. (B) Dual-luciferase assay of AEG-1 WT and MT 3′-UTR with miR-342-3p mimic or miR-342-3p inhibitor in MG63 cells. *p < 0.05 versus NC.
Figure 5.
miR-342-3p regulates AEG-1 expression. RT-qPCR of AEG-1 mRNA expression in MG63 (A) and U2OS (B) cells transfected with miR-342-3p mimic or miR-342-3p inhibitor for 48 h. Western blot of AEG-1 protein expression in MG63 (C) and U2OS (D) cells transfected with miR-342-3p mimic or miR-342-3p inhibitor for 48 h. *p < 0.05 versus NC.
miR-342-3p Inhibits the Wnt and NF-κB Signaling Pathways
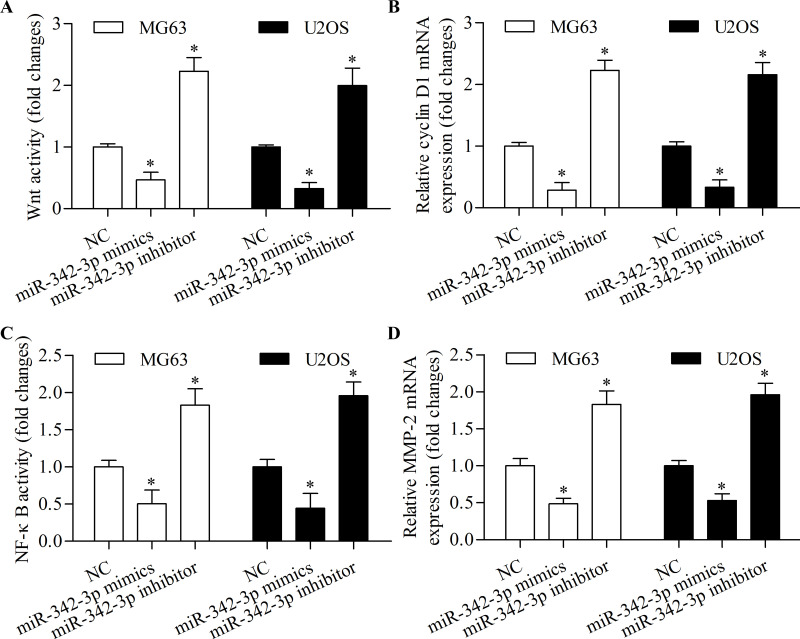
To further determine the molecular mechanism of miR-342-3p in regulating osteosarcoma cell proliferation, migration, and invasion, we detected the role of miR-342-3p on the Wnt and NF-κB signaling pathways. The results showed that miR-342-3p overexpression significantly inhibited Wnt pathway activity (Fig. 6A) and downstream target gene cyclin D1 expression (Fig. 6B). Moreover, miR-342-3p overexpression markedly suppressed NF-κB pathway activity (Fig. 6C) and downstream target gene MMP-2 expression (Fig. 6D). Overall, these results suggest that miR-342-3p inhibits osteosarcoma cell proliferation, migration, and invasion associated with inhibition of the Wnt and NF-κB signaling pathways.
Figure 6.
miR-342-3p inhibits the Wnt and nuclear factor κB (NF-κB) signaling pathways. (A) Wnt luciferase assay in MG63 and U2OS cells transfected with miR-342-3p mimic or miR-342-3p inhibitor and TOPFlash luciferase reporter vector for 48 h. (B) RT-qPCR of cyclin D1 mRNA expression in MG63 and U2OS cells transfected with miR-342-3p mimic or miR-342-3p inhibitor for 48 h. (C) NF-κB luciferase assay in MG63 and U2OS cells transfected with miR-342-3p mimic or miR-342-3p inhibitor and NF-κB reporter vector for 48 h. (D) RT-qPCR of MMP-2 mRNA expression in MG63 and U2OS cells transfected with miR-342-3p mimic or miR-342-3p inhibitor for 48 h. *p < 0.05 versus NC.
Overexpression of AEG-1 Rescues miR-342-3p-Induced Antitumor Effects
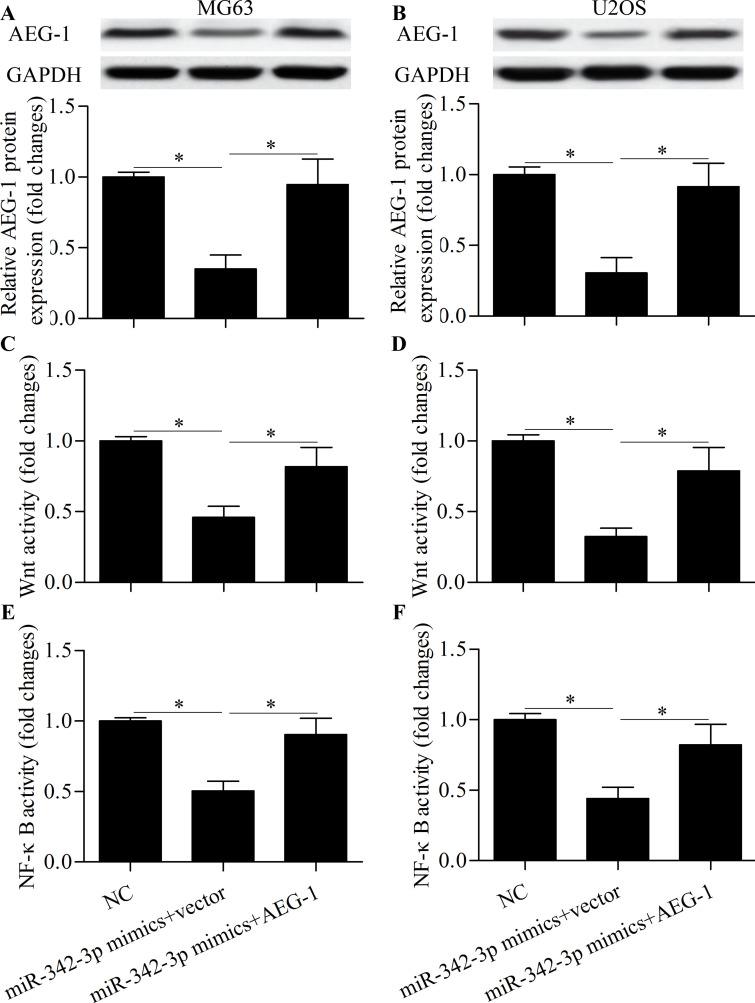
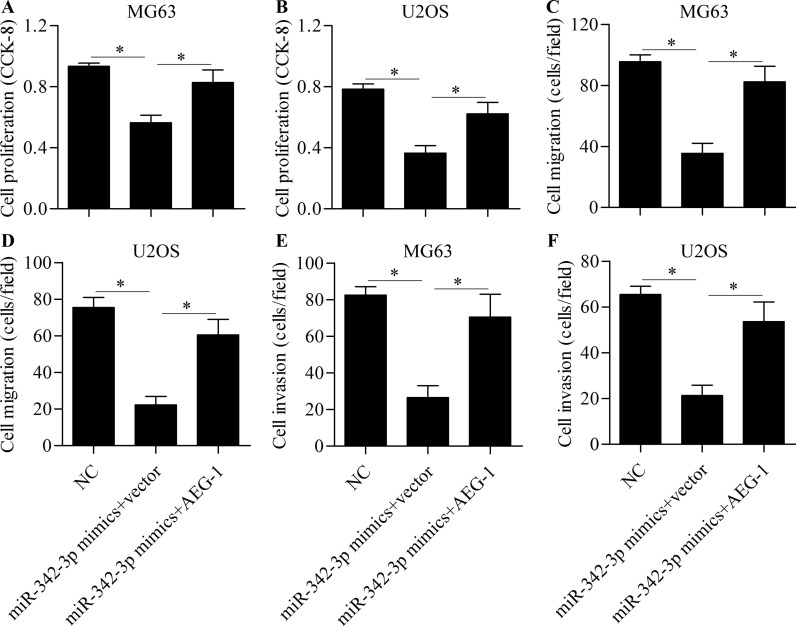
To further investigate whether miR-342-3p inhibits osteosarcoma cell proliferation, migration, and invasion through AEG-1, cells were cotransfected with miR-342-3p mimics and AEG-1 vector. Western blot showed that the reduced expression of AEG-1 induced by miR-342-3p overexpression was markedly restored by AEG-1 vector transfection (Fig. 7A and B). The inhibitory effects of miR-342-3p overexpression on the Wnt (Fig. 7C and D) and NF-κB (Fig. 7E and F) signaling pathways were significantly reversed by AEG-1 overexpression. Furthermore, AEG-1 overexpression partially rescued cell proliferation (Fig. 8A and B), migration (Fig. 8C and D), and invasion (Fig. 8E and F). These results suggest that miR-342-3p inhibits osteosarcoma cell proliferation, migration, and invasion through the downregulation of AEG-1.
Figure 7.
Overexpression of AEG-1 reverses the inhibitory effect of miR-342-3p on the Wnt and NF-κB signaling pathways. Western blot of AEG-1 protein expression in MG63 (A) and U2OS (B) cells transfected with miR-342-3p mimic and AEG-1 vector for 48 h. Wnt luciferase assay in MG63 (C) and U2OS (D) cells transfected with miR-342-3p mimic, AEG-1 vector, and TOPFlash luciferase reporter vector for 48 h. NF-κB luciferase assay in MG63 (E) and U2OS (F) cells transfected with miR-342-3p mimic, AEG-1 vector, and NF-κB reporter vector for 48 h. *p < 0.05.
Figure 8.
Overexpression of AEG-1 rescues miR-342-3p-induced antitumor effects. Cells were transfected with miR-342-3p mimic and AEG-1 vector for 48 h. Cell proliferation of MG63 (A) and U2OS (B) cells was detected by CCK-8. Cell migration of MG63 (C) and U2OS (D) cells was detected by Transwell migration assay. Cell invasion of MG63 (E) and U2OS (F) cells was detected by Transwell invasion assay. *p < 0.05.
DISCUSSION
Altered miRNA expression has been well documented in nearly all human diseases, especially in cancer10,11. miRNAs are involved in regulating proliferation, apoptosis, migration, and invasion8,9. Increasing evidence suggests that miRNAs also participate in the tumorigenesis of osteosarcoma14–17. Therefore, miRNAs have emerged as potential and promising targets for the prognosis, diagnosis, and treatment of osteosarcoma. However, the precise role of miRNAs in osteosarcoma needs to be further studied. In this study, we found that the expression level of miR-342-3p was decreased in osteosarcoma tissue and cell lines. Overexpression of miR-342-3p inhibited osteosarcoma cell proliferation, migration, and invasion. Moreover, AEG-1 was identified as one of the target genes of miR-342-3p, and AEG-1 overexpression abolished the roles of miR-342-3p in osteosarcoma cells. Taken together, our results show that miR-342-3p acts as a tumor suppressor in osteosarcoma progression and development through targeting AEG-1.
miR-342-3p has been reported as a cancer-related miRNA involved in various cancers. miR-342-3p has been suggested to be a predictive biomarker for glioma34, colon cancer35,36, and pancreatic cancer37. Li et al. reported that miR-342-3p inhibits the proliferation, migration, and invasion of human cervical cancer by targeting the forkhead box protein M138. A recent study showed that the long noncoding RNA H19 promotes the expression of forkhead box protein M1 by competitively binding endogenous miR-342-3p in gallbladder cancer39. miR-342-3p inhibits hepatocellular carcinoma cell proliferation by targeting the inhibitor of NF-κB kinase and transforming growth factor-β-activated kinase 1-binding protein 2/3 of the NF-κB signaling pathway40. In non-small cell lung cancer, miR-342-3p is found to be decreased, and the overexpression of miR-342-3p inhibits cell proliferation and invasion through targeting the Ras-related protein Rap-2b33. miR-342-3p also inhibits the proliferation of lung cancer cells through inhibiting Myc transcriptional activity via targeting E2F transcription factor 132. mR-342-3p is decreased in extranodal natural killer/T-cell lymphoma, nasal type, and may contribute to the pathogenesis by regulating T-lymphoma invasion and metastasis-inducing factor 141. These reports suggest an antitumor role for miR-342-3p. Our results showed that miR-342-3p was decreased in osteosarcoma and that the overexpression of miR-342-3p inhibited osteosarcoma proliferation, migration, and invasion. Our data support a tumor suppressive role for miR-342-3p.
AEG-1 is a widely studied oncogene in various cancers18–21. AEG-1 is also implicated in the tumorigenesis of osteosarcoma. AEG-1 is highly expressed in osteosarcoma tissue, and the overexpression of AEG-1 is strongly associated with clinical stage, metastasis, and poor survival of osteosarcoma patients27. Similarly, Tang et al. reported that the overexpression of AEG-1 was significantly associated with metastasis and poor survival of osteosarcoma patients and that the knockdown of AEG-1 inhibited proliferation, migration, and invasion of osteosarcoma cells28. AEG-1 promotes the carcinogenesis and invasiveness of osteosarcoma by upregulating MMP-227. Liu et al. reported that AEG-1 promoted osteosarcoma metastasis and chemoresistance by endothelin-1/endothelin A receptor signaling29. AEG-1 has been reported to promote osteosarcoma cell invasion through activation of Jun N-terminal kinase/MMP-2 signaling30. Furthermore, Wnt/cyclin D1 and NF-κB/MMP-2 signaling are also involved in AEG-1-mediated carcinogenesis and invasiveness of osteosarcoma31,42. Thus, AEG-1 serves as a potential therapeutic target for osteosarcoma treatment. In this study, we identified that AEG-1 was a target gene of miR-342-3p. Our findings showed that overexpression of miR-342-3p inhibited proliferation, migration, and invasion of osteosarcoma cells through inhibiting AEG-1 as well as Wnt/cyclin D1 and NF-κB/MMP-2 signaling. Our study suggests that miR-342-3p acts as a tumor suppressor gene, and the decreased expression of miR-342-3p may contribute to the progression and metastasis of osteosarcoma through AEG-1-mediated oncogenic signaling.
A growing body of evidence has suggested that AEG-1 undergoes epigenetic regulation by miRNAs during cancer development and progression43,44. Numerous tumor suppressive miRNAs such as miR-137 and miR-375 block tumorigenesis by targeting AEG-143,45. Thus, inhibiting AEG-1 by miRNAs represents a promising therapeutic strategy for cancer treatment. Interestingly, a recent study revealed that AEG-1 is epigenetically regulated by miR-506 in osteosarcoma cells and that overexpression of miR-506 inhibits tumor growth of osteosarcoma through downregulation of AEG-131. In the present study, we showed that miR-342-3p was a novel regulator for AEG-1 in osteosarcoma. We found that inhibiting AEG-1 by miR-342-3p suppressed the proliferation, migration, and invasion of osteosarcoma cells. Our study suggests that inhibiting AEG-1 by miR-342-3p may be a promising strategy for the treatment of osteosarcoma.
In conclusion, our study reveals that miR-342-3p functions as a tumor suppressor through inhibiting cancer cell proliferation, migration, and invasion by targeting AEG-1 in human osteosarcoma. These findings provide novel insights into understanding the molecular pathogenesis of osteosarcoma. Targeting miR-332-3p may be a potential and promising strategy for the development of novel miRNA-based anticancer therapies for osteosarcoma.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Arndt CA, Crist WM. Common musculoskeletal tumors of childhood and adolescence. N Engl J Med. 1999;341:342–52. [DOI] [PubMed] [Google Scholar]
- 2. Botter SM, Neri D, Fuchs B. Recent advances in osteosarcoma. Curr Opin Pharmacol. 2014;16:15–23. [DOI] [PubMed] [Google Scholar]
- 3. Yang J, Zhang W. New molecular insights into osteosarcoma targeted therapy. Curr Opin Oncol. 2013;25:398–406. [DOI] [PubMed] [Google Scholar]
- 4. Rainusso N, Wang LL, Yustein JT. The adolescent and young adult with cancer: State of the art—Bone tumors. Curr Oncol Rep. 2013;15:296–307. [DOI] [PubMed] [Google Scholar]
- 5. Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116:281–97. [DOI] [PubMed] [Google Scholar]
- 6. Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: MicroRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–34. [DOI] [PubMed] [Google Scholar]
- 7. Kim YK, Yu J, Han TS, Park SY, Namkoong B, Kim DH, Hur K, Yoo MW, Lee HJ, Yang HK, Kim VN. Functional links between clustered microRNAs: Suppression of cell-cycle inhibitors by microRNA clusters in gastric cancer. Nucleic Acids Res. 2009;37:1672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsai HC, Su HL, Huang CY, Fong YC, Hsu CJ, Tang CH. CTGF increases matrix metalloproteinases expression and subsequently promotes tumor metastasis in human osteosarcoma through down-regulating miR-519d. Oncotarget 2014;5:3800–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu Y, Li F, Xu T, Sun J. MiRNA-497 negatively regulates the growth and motility of chondrosarcoma cells by targeting cdc25A. Oncol Res. 2016;23:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Li Y, Deng X, Zeng X, Peng X. The role of mir-148a in cancer. J Cancer 2016;7:1233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Svoronos AA, Engelman DM, Slack FJ. OncomiR or tumor suppressor? The duplicity of microRNAs in cancer. Cancer Res. 2016;76:3666–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krutzfeldt J. Strategies to use microRNAs as therapeutic targets. Best Pract Res Clin Endocrinol Metab. 2016;30:551–61. [DOI] [PubMed] [Google Scholar]
- 13. Shah MY, Ferrajoli A, Sood AK, Lopez-Berestein G, Calin GA. MicroRNA therapeutics in cancer—An emerging concept. EBioMedicine 2016;12:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liang W, Gao B, Fu P, Xu S, Qian Y, Fu Q. The miRNAs in the pathgenesis of osteosarcoma. Front Biosci. (Landmark Ed) 2013;18:788–94. [DOI] [PubMed] [Google Scholar]
- 15. Kushlinskii NE, Fridman MV, Braga EA. Molecular mechanisms and microRNAs in osteosarcoma pathogenesis. Biochemistry (Mosc) 2016;81:315–28. [DOI] [PubMed] [Google Scholar]
- 16. Sampson VB, Yoo S, Kumar A, Vetter NS, Kolb EA. MicroRNAs and potential targets in osteosarcoma: Review. Front Pediatr. 2015;3:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang J, Yan YG, Wang C, Zhang SJ, Yu XH, Wang WJ. MicroRNAs in osteosarcoma. Clin Chim Acta 2015;444:9–17. [DOI] [PubMed] [Google Scholar]
- 18. Shi X, Wang X. The role of MTDH/AEG-1 in the progression of cancer. Int J Clin Exp Med. 2015;8:4795–807. [PMC free article] [PubMed] [Google Scholar]
- 19. Hu G, Wei Y, Kang Y. The multifaceted role of MTDH/AEG-1 in cancer progression. Clin Cancer Res. 2009;15:5615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Emdad L, Sarkar D, Su ZZ, Lee SG, Kang DC, Bruce JN, Volsky DJ, Fisher PB. Astrocyte elevated gene-1: Recent insights into a novel gene involved in tumor progression, metastasis and neurodegeneration. Pharmacol Ther. 2007;114:155–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Emdad L, Das SK, Hu B, Kegelman T, Kang DC, Lee SG, Sarkar D, Fisher PB. AEG-1/MTDH/LYRIC: A promiscuous protein partner critical in cancer, obesity, and CNS diseases. Adv Cancer Res. 2016;131:97–132. [DOI] [PubMed] [Google Scholar]
- 22. Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao W, Volsky DJ, Fisher PB. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene 2002;21: 3592–602. [DOI] [PubMed] [Google Scholar]
- 23. Li C, Wu X, Zhang W, Li J, Liu H, Hao M, Wang J, Zhang H, Yang G, Sheng S, Sun Y, Long J, Hu X, Hu C, Li L, Zheng J. AEG-1 promotes metastasis through downstream AKR1C2 and NF1 in liver cancer. Oncol Res. 2014;22:203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Robertson CL, Srivastava J, Siddiq A, Gredler R, Emdad L, Rajasekaran D, Akiel M, Shen XN, Guo C, Giashuddin S, Wang XY, Ghosh S, Subler MA, Windle JJ, Fisher PB, Sarkar D. Genetic deletion of AEG-1 prevents hepatocarcinogenesis. Cancer Res. 2014;74:6184–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Emdad L, Sarkar D, Lee SG, Su ZZ, Yoo BK, Dash R, Yacoub A, Fuller CE, Shah K, Dent P, Bruce JN, Fisher PB. Astrocyte elevated gene-1: A novel target for human glioma therapy. Mol Cancer Ther. 2010;9:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang Y, Li LP. Progress of cancer research on astrocyte elevated gene-1/metadherin (review). Oncol Lett. 2014;8:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang F, Ke ZF, Sun SJ, Chen WF, Yang SC, Li SH, Mao XP, Wang LT. Oncogenic roles of astrocyte elevated gene-1 (AEG-1) in osteosarcoma progression and prognosis. Cancer Biol Ther. 2011;12:539–48. [DOI] [PubMed] [Google Scholar]
- 28. Tang J, Shen L, Yang Q, Zhang C. Overexpression of metadherin mediates metastasis of osteosarcoma by regulating epithelial-mesenchymal transition. Cell Prolif. 2014;47:427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu B, Wu Y, Peng D. Astrocyte elevated gene-1 regulates osteosarcoma cell invasion and chemoresistance via endothelin-1/endothelin A receptor signaling. Oncol Lett. 2013;5:505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang F, Ke ZF, Wang R, Wang YF, Huang LL, Wang LT. Astrocyte elevated gene-1 (AEG-1) promotes osteosarcoma cell invasion through the JNK/c-Jun/MMP-2 pathway. Biochem Biophys Res Commun. 2014;452:933–9. [DOI] [PubMed] [Google Scholar]
- 31. Yao J, Qin L, Miao S, Wang X, Wu X. Overexpression of miR-506 suppresses proliferation and promotes apoptosis of osteosarcoma cells by targeting astrocyte elevated gene-1. Oncol Lett. 2016;12:1840–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tai MC, Kajino T, Nakatochi M, Arima C, Shimada Y, Suzuki M, Miyoshi H, Yatabe Y, Yanagisawa K, Takahashi T. MiR-342-3p regulates MYC transcriptional activity via direct repression of E2F1 in human lung cancer. Carcinogenesis 2015;36:1464–73. [DOI] [PubMed] [Google Scholar]
- 33. Xie X, Liu H, Wang M, Ding F, Xiao H, Hu F, Hu R, Mei J. miR-342-3p targets RAP2B to suppress proliferation and invasion of non-small cell lung cancer cells. Tumour Biol. 2015;36:5031–8. [DOI] [PubMed] [Google Scholar]
- 34. Wang Q, Li P, Li A, Jiang W, Wang H, Wang J, Xie K. Plasma specific miRNAs as predictive biomarkers for diagnosis and prognosis of glioma. J Exp Clin Cancer Res. 2012;31:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tao K, Yang J, Guo Z, Hu Y, Sheng H, Gao H, Yu H. Prognostic value of miR-221-3p, miR-342-3p and miR-491-5p expression in colon cancer. Am J Transl Res. 2014;6:391–401. [PMC free article] [PubMed] [Google Scholar]
- 36. Jung CK, Jung SH, Yim SH, Jung JH, Choi HJ, Kang WK, Park SW, Oh ST, Kim JG, Lee SH, Chung YJ. Predictive microRNAs for lymph node metastasis in endoscopically resectable submucosal colorectal cancer. Oncotarget 2016;7:32902–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee KH, Lee JK, Choi DW, Do IG, Sohn I, Jang KT, Jung SH, Heo JS, Choi SH, Lee KT. Postoperative prognosis prediction of pancreatic cancer with seven microRNAs. Pancreas 2015;44:764–8. [DOI] [PubMed] [Google Scholar]
- 38. Li XR, Chu HJ, Lv T, Wang L, Kong SF, Dai SZ. miR-342-3p suppresses proliferation, migration and invasion by targeting FOXM1 in human cervical cancer. FEBS Lett. 2014;588:3298–307. [DOI] [PubMed] [Google Scholar]
- 39. Wang SH, Ma F, Tang ZH, Wu XC, Cai Q, Zhang MD, Weng MZ, Zhou D, Wang JD, Quan ZW. Long non-coding RNA H19 regulates FOXM1 expression by competitively binding endogenous miR-342-3p in gallbladder cancer. J Exp Clin Cancer Res. 2016;35:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao L, Zhang Y. miR-342-3p affects hepatocellular carcinoma cell proliferation via regulating NF-kappaB pathway. Biochem Biophys Res Commun. 2015;457:370–7. [DOI] [PubMed] [Google Scholar]
- 41. Huang H, Fan L, Zhan R, Wu S, Niu W. Expression of microRNA-10a, microRNA-342-3p and their predicted target gene TIAM1 in extranodal NK/T-cell lymphoma, nasal type. Oncol Lett. 2016;11:345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Z, Cao CJ, Huang LL, Ke ZF, Luo CJ, Lin ZW, Wang F, Zhang YQ, Wang LT. EFEMP1 promotes the migration and invasion of osteosarcoma via MMP-2 with induction by AEG-1 via NF-kappaB signaling pathway. Oncotarget 2015;6:14191–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. He XX, Chang Y, Meng FY, Wang MY, Xie QH, Tang F, Li PY, Song YH, Lin JS. MicroRNA-375 targets AEG-1 in hepatocellular carcinoma and suppresses liver cancer cell growth in vitro and in vivo. Oncogene 2012;31:3357–69. [DOI] [PubMed] [Google Scholar]
- 44. Nohata N, Hanazawa T, Kikkawa N, Mutallip M, Sakurai D, Fujimura L, Kawakami K, Chiyomaru T, Yoshino H, Enokida H, Nakagawa M, Okamoto Y, Seki N. Tumor suppressive microRNA-375 regulates oncogene AEG-1/MTDH in head and neck squamous cell carcinoma (HNSCC). J Hum Genet. 2011;56:595–601. [DOI] [PubMed] [Google Scholar]
- 45. Yan JW, Lin JS, He XX. The emerging role of miR-375 in cancer. Int J Cancer 2014;135:1011–8. [DOI] [PubMed] [Google Scholar]