Abstract
Transforming growth factor-β (TGF-β) and ERK signaling have been implicated in various human cancers including hepatocellular carcinoma, but the underlying mechanism remains largely unclear. In this study, we aimed to explore the role of ERK1/2 in the regulation of TGF-β’s promoting and suppressive activities in HCC cells. Our data showed that treatment with TGF-β1 enhanced invasion and epithelial–mesenchymal transition (EMT) in HCC HepG2 cells, accompanied with increased MMP9 production and activation of Smad2/3 and ERK1/2, but inhibited tumor cell proliferation. These effects were eliminated by treatment with SB431542, a TGF-β inhibitor. Afterward, treatment with the MEK1/2 inhibitor U0126 reduced the TGF-β1-induced invasion and vimentin and MMP9 secretion in HepG2 cells, without affecting the inhibitory effects of TGF-β1 on HepG2 cell proliferation. Moreover, inhibition of Smad2/3 expression attenuated TGF-β1-induced cell invasion, ERK1/2 phosphorylation, and MMP9 production in HepG2 cells. However, knockdown of Slug only reduced cell invasion but did not affect ERK1/2 activation and MMP9 secretion in HepG2 cells. These data indicate that TGF-β1 activates ERK1/2 in HepG2 cells through the Smad2/3 pathway but not the Slug pathway. In summary, our study demonstrates that inhibition of ERK1/2 signaling attenuates the promoting effects of TGF-β1 on the metastatic phenotypes of HCC cells without affecting its suppressive effects on HCC cell proliferation. Therefore, we suggest that ERK1/2 may be used as a molecular target for the treatment of TGF-β-responsive HCC.
Key words: Hepatocellular carcinoma (HCC), Transforming growth factor-β1 (TGF-β1), ERK1/2, Migration, Epithelial–mesenchymal transition (EMT)
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignant cancers with a rapidly increased incidence, causing a large number of deaths worldwide1,2. Although great efforts have been made toward surgical resection, radiotherapy, and chemotherapy, the prognosis for patients with advanced HCC remains poor2. Revealing the molecular mechanism underlying the development and progression of HCC may be of benefit for developing novel therapeutic strategies for HCC.
Transforming growth factor-β1 (TGF-β1), a secreted ligand of the TGF-β superfamily of proteins, can bind various TGF-β receptors and lead to recruitment and activation of SMAD family transcription factors that regulate gene expression3,4. In addition, TGF-β1 can regulate the expression and activation of other growth factors, such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α)3,5. Through regulating the expression of various genes, TGF-β1 participates in diverse cellular biological processes, such as embryogenesis, cell proliferation, differentiation, inflammation, and so forth4,6. It has been demonstrated that the expression of TGF-β1 is increased during liver injury, and TGF-β1 plays a promoting role in liver fibrosis7. At the early stage of tumor development, TGF-β1 acts as a tumor suppressor through inhibition of cell proliferation and induction of cell apoptosis8,9. During the malignant progression of cancer, however, TGF-β1 plays a tumor-promoting role in tumor invasion and metastasis by inducing epithelial–mesenchymal transition (EMT)8. Moreover, TGF-β1 generally functions through Smad2/3 signaling, but several other signaling molecules, including ERK1/2, also play key roles, which is associated with the tumor-promoting activity of TGF-β110–12.
In fact, the TGF-β1 signaling pathway plays a dual role in liver cancer, and it acts as a tumor promoter or suppressor depending on the cellular context13,14. Several studies demonstrate that TGF-β1 is significantly upregulated in HCC tissues compared with paracarcinoma tissues, and a high expression of TGF-β1 is associated with advanced clinical stage as well as poor survival time in HCC patients15,16. TGF-β1 was also found to promote HCC cell migration, invasion, and EMT17. Moreover, the expression of ERK was found to be significantly upregulated in HCC tissues compared with paracarcinoma tissues, and the activity of ERK in paracarcinoma tissues was higher than that in normal liver tissues18. A recent study demonstrated that blocking of the TGF-β1/ERK2 pathway could inhibit EMT and hepatic carcinogenesis19. However, the underlying mechanism remains to be fully uncovered.
Therefore, the present study aimed to explore the exact role of ERK signaling in the regulation of TGF-β’s tumor-promoting or suppressive activities in HCC, as well as the underlying mechanism.
MATERIALS AND METHODS
Cell Culture
An HCC HepG2 cell line was obtained from the Cell Bank of Xiangya Medical School (Central South University, Changsha, P.R. China) and cultured in DMEM (Thermo Fisher, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS; Thermo Fisher) in a humidified incubator (Thermo Fisher) containing 5% CO2 at 37°C.
Cell Treatment
HepG2 cells (10,000 cells/well) in DMEM with 10% FBS were seeded into a 96-well plate and treated with 5 ng/ml TGF-β1 (R&D Systems, Minneapolis, MN, USA), 10 μM SB431542 (Cayman, Ann Arbor, MI, USA), 1 μM MEK1/2 inhibitor U0126 (Tocris, Bristol, UK), or a combination of TGF-β1 and SB431542 or U0126. In the control group, HepG2 cells were treated with vehicle (4 μM HCl, 0.001% BSA, and 0.025% DMSO).
Cell Transfection
For cell transfection, Lipofectamine 2000 (Thermo Fisher) was used according to the manufacturer’s instructions. In brief, MMP9 siRNA, Smad2/3 siRNA, Slug siRNA, or nonspecific siRNA (NC siRNA) was transfected into HepG2 cells. After 48 h of transfection, the protein expression analysis was performed using Western blot.
MTT Assay
An MTT assay was used to examine cell proliferation. HepG2 cells in each group were added to 0.5 g/L MTT (Sigma-Aldrich, St. Louis, MO, USA) and incubated at 37°C for 0, 24, 48, and 72 h. The medium was then removed, and 50 μl of DMSO was added. After incubation at 37°C for 10 min, the optical density was measured using the UV-3600 spectrophotometer (Shimadzu, Kyoto, Japan).
Wound-Healing Assay
HepG2 cells in DMEM with 0.1% FBS were seeded into a 24-well plate and treated with TGF-β1 with or without SB431542 for 24 h. In the control group, HepG2 cells were treated with vehicle (4 μM HCl, 0.001% BSA, and 0.025% DMSO) for 24 h. Wounds were then created using a plastic scriber (about 1-mm width), and cells were washed with DPBS and then added to DMEM with 0.1% FBS containing TGF-β1 with or without SB431542. After incubation at 37°C for 48 h, cells were photographed under an inverted microscope (IX71; Olympus, Tokyo, Japan).
Transwell Assay
Transwell assay was used to examine cell invasion using Transwell chambers (BD Biosciences, San Jose, CA, USA) precoated with Matrigel (BD). HepG2 cell suspension (5 × 105 cells/ml) in each group was prepared in DMEM, 300 μl of which was added into the upper chamber. DMEM (500 μl) with 10% FBS was added into the lower chamber. After incubation in 37°C for 24 h, a cotton-tipped swab was used to wipe out those HepG2 cells that did not go through the pores. The filter was then fixed in 90% alcohol and stained with crystal violet (Sigma-Aldrich). Cells that went through the pores were counted and photographed under an inverted microscope (IX71; Olympus).
Real-Time qPCR
Total RNA was extracted from HepG2 cells using TRIzol reagent (Thermo Fisher), which was then converted into cDNA using a reverse transcription kit (Thermo Fisher) according to the manufacturer’s instructions. For detection of mRNA expression, real time (RT)-PCR was conducted using standard SYBR Green RT-PCR Kit (Takara, Dalian, P.R. China) on an ABI 7300 Plus Thermocycler (Thermo Fisher) according to the manufacturer’s instructions. The reaction condition was 95°C for 3 min, followed by 40 cycles of 95°C for 30 s, and 60°C for 30 s. GAPDH was used as an internal reference. The expression analysis was conducted using the 2−ΔΔCt method.
Western Blot
HepG2 cells in each group were lysed in RIPA buffer. The lysates were centrifuged at 12,000 × g for 30 min at 4°C. The protein concentration was then determined using the BCA Protein Assay Kit (Beyotime Biotechnology, Shanghai, P.R. China) according to the manufacturer’s instructions. The protein (50 μg) was separated in 12% SDS-PAGE gel and then transferred onto a polyvinylidene fluoride (PVDF) membrane (Thermo Fisher). The PVDF membrane was blocked in 5% nonfat milk (Yili, Beijing, P.R. China) at room temperature for 3 h, and then incubated with rabbit anti-human phospho (p)-Smad2, total (t)-Smad2, p-ERK1/2, t-ERK1/2, E-cadherin, N-cadherin, vimentin, Slug, MMP9, and GAPDH, followed by goat anti-rabbit secondary antibody (all from Abcam, Cambridge, MA, USA). The immunoblots on the membrane were visualized using Enhanced Chemiluminescence Kit (Thermo Fisher) and recorded by G:Box Chemi XL system (Syngene, Cambridge, UK).
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA was conducted to examine the production of inflammatory cytokines in dorsal horns of rats in each group. Human MMP9 ELISA Kit Yearthbio Company Limited (www.yearthbio.com) was used to measure the secretion levels of MMP9 according to the manufacturer’s instructions. The optical density at 450 nm was detected using the UV-3600 spectrophotometer.
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM) of three independent experiments. Statistical analysis was conducted using SPSS 19.0. Comparisons of two groups were performed using Student’s t-test and of more than two groups by one-way ANOVA. The value of p < 0.05 was considered as statistically significant.
RESULTS
TGF-β1 Promotes Invasion While Inhibiting Proliferation of HCC Cells
It has been demonstrated that TGF-β1 participates in tumor invasion and metastasis. In this study, HCC HepG2 cells were treated with TGF-β1 for 24 h. Treatment with TGF-β1 significantly promoted HepG2 cell invasion when compared to the control group (Fig. 1A). To further confirm these findings, the TGF-β inhibitor SB431542 was added. We found that treatment with SB431542 abolished the promoting effects of TGF-β1 on HepG2 cell invasion (Fig. 1A). We then studied the effects of TGF-β1 on HepG2 cell proliferation. Our data indicated that treatment with TGF-β1 significantly reduced HepG2 cell proliferation compared to the control group, which was also abolished by SB431542 treatment (Fig. 1B).
Figure 1.
Transforming growth factor-β1 (TGF-β1) promotes invasion while inhibiting the proliferation of hepatocellular carcinoma (HCC) cells. HepG2 cells were treated with TGF-β1 with or without SB431542 for 24 h. (A) Transwell assay and (B) MTT assay were conducted to examine cell invasion and proliferation, respectively. Nontreated cells were used as the control group. **p < 0.01.
TGF-β1 Induces EMT and Upregulates MMP9 in HCC Cells
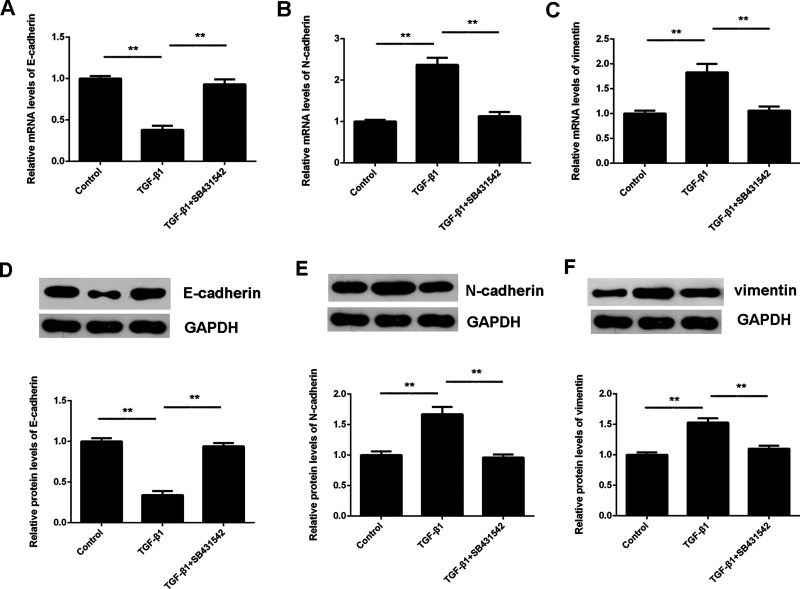
It has been demonstrated that EMT is important for tumor invasion and metastasis. Therefore, we further studied the EMT in HepG2 cells treated with TGF-β1 or TGF-β1 and SB431542, respectively. Several key factors associated with EMT, including E-cadherin, N-cadherin, and vimentin, were evaluated using RT-PCR and Western blot. The mRNA and protein levels of E-cadherin were significantly reduced, whereas N-cadherin and vimentin were significantly upregulated in HepG2 cells treated with TGF-β1 (Fig. 2). These effects of TGF-β1 were abolished by SB431542 treatment (Fig. 2). These data confirm that TGF-β1 could induce EMT in HepG2 cells.
Figure 2.
TGF-β1 induces epithelial–mesenchymal transition (EMT) in HCC cells. HepG2 cells were treated with TGF-β1 with or without SB431542 for 24 h. (A–C) Real-time (RT)-PCR and (D, E) Western blot were used to examine the mRNA and protein levels of E-cadherin, N-cadherin, and vimentin, respectively. GAPDH was used as an internal reference. Nontreated cells were used as the control group. **p < 0.01.
In addition, MMP9 is important for tumor invasion and metastasis, and thus we examined its protein expression and secretion in HepG2 cells in each group using Western blot and ELISA. Our data showed that treatment with TGF-β1 not only promoted the protein expression of MMP9 but also increased its secretion (Fig. 3A and B). Similarly, these effects of TGF-β1 were abolished by SB431542 treatment (Fig. 3A and B). To further study the role of MMP9 in TGF-β1-induced HepG2 cell invasion, we used MMP9 siRNA to knock down its expression. NC siRNA was used as a negative control. The invasion of HepG2 cells was significantly reduced in the TGF-β1 + MMP9 siRNA group compared with the TGF-β1 + NC siRNA group (Fig. 3C). To further confirm these findings, Western blot was conducted to examine the MMP9 protein expression, and the result showed that the protein and secretion levels of MMP9 were significantly lower in the TGF-β1 + MMP9 siRNA group, compared with the TGF-β1 + NC siRNA group (Fig. 3D and E).
Figure 3.
TGF-β1 upregulates MMP9 in HCC cells. HepG2 cells were treated with TGF-β1 with or without SB431542 for 24 h. Nontreated cells were used as the control group. (A) Western blot and (B) enzyme-linked immunosorbent assay (ELISA) were used to examine the protein expression and secretion levels of MMP9, respectively. Afterward, MMP9 siRNA or nonspecific siRNA (NC siRNA) were transfected into HepG2 cells, which were then treated with TGF-β1 for 24 h. (C) Transwell assay was conducted to examine the cell invasion. (D) Western blot and (E) ELISA were used to examine the protein expression and secretion levels of MMP9, respectively. **p < 0.01.
ERK1/2 Is Involved in TGF-β1-Induced HepG2 Cell Invasion
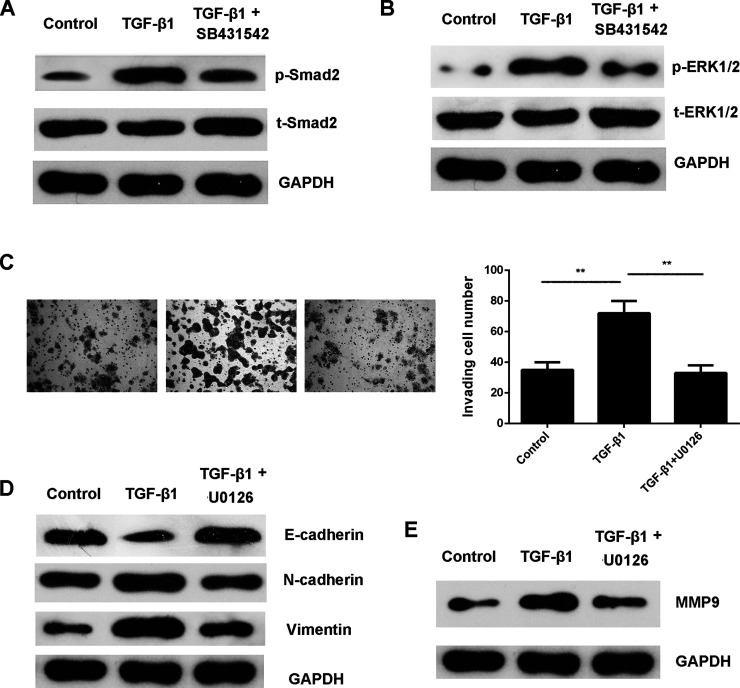
Smad signaling has been demonstrated to be involved in EMT. Our data showed that the p-Smad2 levels were indeed increased in HepG2 cells treated with TGF-β1, which was also inhibited by SB431542 (Fig. 4A). Moreover, ERK1/2 is a downstream effector of Smad signaling. Therefore, we studied the activity of ERK signaling in HepG2 cells in each group. The p-ERK1/2 levels were also increased in HepG2 cells treated with TGF-β1, but these effects were abolished by SB431542 treatment (Fig. 4B).
Figure 4.
ERK1/2 is involved in TGF-β1-induced HepG2 cell invasion. HepG2 cells were treated with TGF-β1 with or without SB431542 for 24 h. Western blot was then used to examine the protein levels of (A) phospho (p)-Smad2 and total (t)-Smad2, as well as (B) p-ERK1/2 and t-ERK1/2, respectively. GAPDH was used as an internal reference. HepG2 cells were then treated with TGF-β1 with or without U0126 for 24 h. (C) Transwell assay was conducted to examine cell invasion. Western blot was used to examine the protein levels of (D) EMT-related genes and (E) MMP9. GAPDH was used as an internal reference. Nontreated cells were used as the control group. **p < 0.01.
We then investigated whether the MEK/ERK pathway was involved in TGF-β1-induced EMT and invasion of HepG2 cells. The MEK1/2 inhibitor U0126 was used. Our data showed that treatment with U0126 suppressed TGF-β1-induced HepG2 cell invasion, accompanied with downregulation of EMT and MMP9 (Fig. 4C–E). These findings suggest that TGF-β1 promotes HepG2 cell invasion by inducing EMT and MMP9 production, at least partly, through activation of ERK1/2 signaling.
ERK1/2 Is Not Involved in the TGF-β1-Induced Inhibition of HCC Cell Proliferation
Next, we studied whether ERK1/2 is involved in the suppressive effects of TGF-β1 on HCC cell proliferation by conducting an MTT assay. Our data showed that treatment with TGF-β1 significantly reduced HepG2 cell proliferation when compared to the control group. However, treatment with U0126 did not affect the suppressive effects of TGF-β1 on HepG2 cell proliferation (Fig. 5). These findings suggest that TGF-β1 could inhibit HCC cell proliferation in an ERK1/2-independent manner. Therefore, inhibition of ERK1/2 signaling could repress the promoting effects of TGF-β1 on HCC cell invasion without affecting its antiproliferation activity.
Figure 5.
ERK1/2 is not involved in TGF-β1-induced inhibition of HCC cell proliferation. HepG2 cells were treated with TGF-β1 with or without U0126 for 24 h. An MTT assay was used to examine cell proliferation. **p < 0.01.
TGF-β1 Activates ERK1/2 in HepG2 Cells Through the Smad2/3 Pathway but not the Slug Pathway
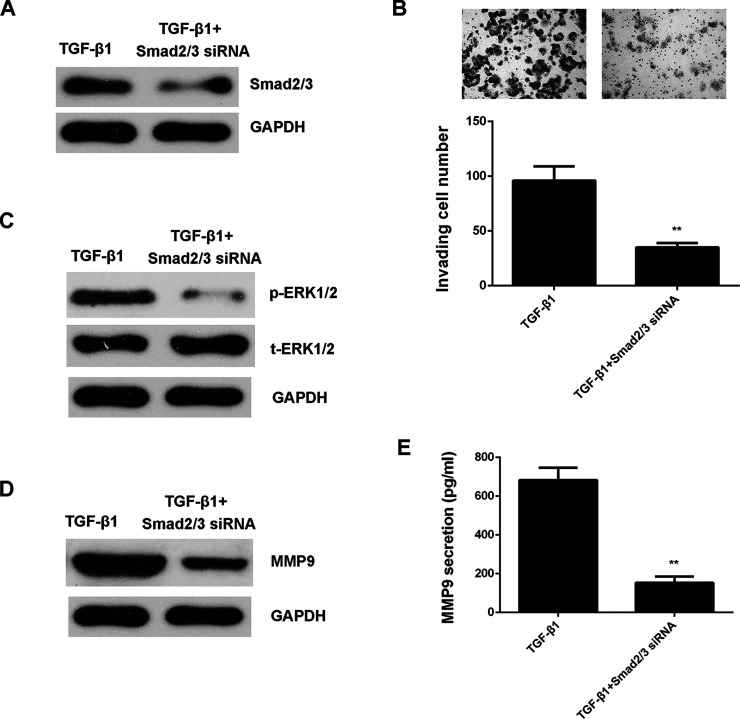
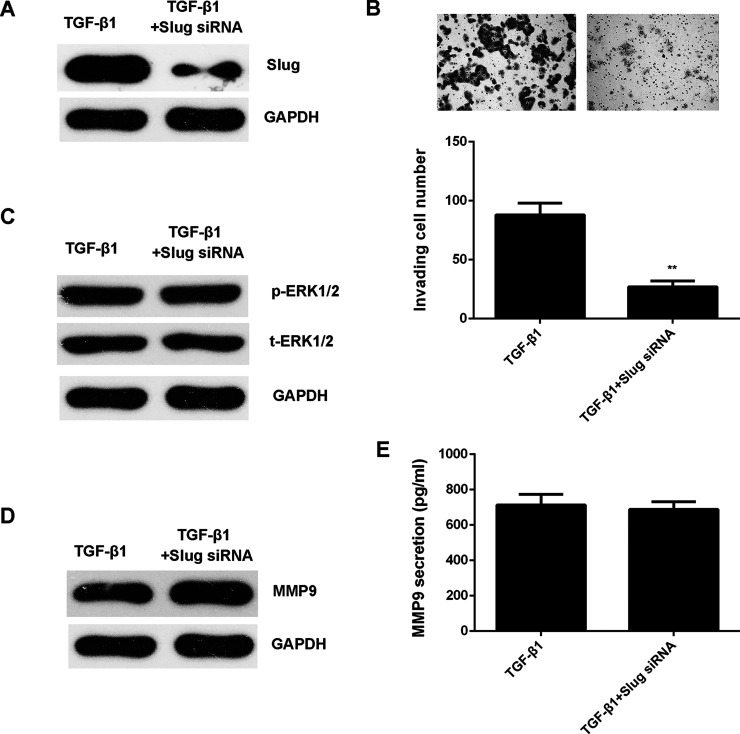
As Smad and Slug are two key regulators downstream of TGF-β1, we then studied whether they participated in TGF-β1-induced cell invasion and ERK1/2 activation in HepG2 cells. Smad2/3- and Slug-specific siRNAs were used to knock down their expression in HepG2 cells. Our data showed that inhibition of Smad2/3 expression attenuated TGF-β1-induced cell invasion, ERK1/2 phosphorylation, and MMP9 expression and production in HepG2 cells (Fig. 6). However, knockdown of Slug only reduced cell invasion and did not affect ERK1/2 activation and MMP9 secretion in HepG2 cells (Fig. 7). These data indicate that TGF-β1 activates ERK1/2 in HepG2 cells through the Smad2/3 pathway but not the Slug pathway.
Figure 6.
TGF-β1 activates ERK1/2 in HepG2 cells through the Smad2/3 pathway. Smad2/3 siRNA or NC siRNA was transfected into HepG2 cells, which were then treated with TGF-β1 for 24 h. (A) Western blot was used to examine the protein levels of Smad2/3. GAPDH was used as an internal reference. (B) Transwell assay was conducted to examine the cell invasion. (C) Western blot was used to examine the protein levels of p-ERK1/2 and t-ERK1/2, respectively. GAPDH was used as an internal reference. (D) Western blot and (E) ELISA were used to examine the protein expression and secretion levels of MMP9, respectively. **p < 0.01.
Figure 7.
TGF-β1 activates ERK1/2 in HepG2 cells not through the Slug pathway. Slug siRNA or NC siRNA was transfected into HepG2 cells, which were then treated with TGF-β1 for 24 h. (A) Western blot was used to examine the protein levels of Slug. GAPDH was used as an internal reference. (B) Transwell assay was conducted to examine cell invasion. (C) Western blot was used to examine the protein levels of p-ERK1/2 and t-ERK1/2, respectively. GAPDH was used as an internal reference. (D) Western blot and (E) ELISA were used to examine the protein expression and secretion levels of MMP9, respectively. **p < 0.01.
DISCUSSION
The effect of ERK signaling on the tumor-promoting or -suppressive activities of TGF-β1 in HCC, as well as the underlying mechanism, remains unclear. As TGF-β1 inhibits cell proliferation and induces cell apoptosis in the early stage of carcinogenesis while acting as a tumor promoter through enhancing tumor invasion and metastasis in later stages8,9, revealing the molecular mechanism underlying the contradicting activities of TGF-β may help inhibit the malignant phenotype of TGF-β1-responsive cancer while keeping the desirable activity of TGF-β. In this study, we showed that TGF-β1 promoted invasion and EMT in HepG2 cells, accompanied with increased MMP9 production and activation of Smad2/3 and ERK1/2, but inhibited tumor cell proliferation. Next, treatment with the MEK1/2 inhibitor U0126 reduced TGF-β1-induced invasion, EMT, and MMP9 production in HepG2 cells without affecting the inhibitory effects of TGF-β1 on tumor cell proliferation. In addition, inhibition of Smad2/3 expression attenuated TGF-β1-induced cell invasion, ERK1/2 phosphorylation, and MMP9 production in HepG2 cells, while knockdown of Slug only reduced cell invasion but did not affect ERK1/2 activation and MMP9 secretion in HepG2 cells, indicating that TGF-β1 activates ERK1/2 in HepG2 cells through the Smad2/3 pathway but not the Slug pathway.
ERK, an important member of the MAPK super family, plays a key role in the Ras/Raf/MEK/ERK signaling pathway and participates in the regulation of many cellular biological processes, such as cell proliferation, apoptosis, and motility20–23. ERK1/2, two subtypes of highly conservative serine/threonine kinase, could be activated by MEK1/2, which will further cause the phosphorylation of many downstream proteins23. For instance, ERK1/2 can phosphorylate several important transcription factors including c-Jun, c-Myc, ribosomal S6 kinase, and NF-κB, and thus initiate their transcription regulation function21. Previous studies have demonstrated that the activation of ERK1/2 plays a role in TGF-β-induced cell invasion and EMT24,25. For instance, Xie et al. reported that inhibition of ERK1/2 signaling suppressed the EMT induced by TGF-β in NSCLC cells25. Moreover, both TGF-β and ERK1/2 have been implicated in the regulation of the malignant phenotypes of HCC cells26. However, the link between TGF-β and ERK1/2 in HCC as well as the detailed regulatory mechanism remain largely unclear. Here we found that TGF-β1 showed antiproliferative activity in HepG2 cells but could promote HepG2 cell invasion, probably through inducing EMT and MMP9 production, which is at least partly attributed to the activation of ERK1/2. More importantly, inhibition of ERK signaling only inhibited the TGF-β1-induced HCC cell invasion but did not affect the suppressive effects of TGF-β1 on HCC cell proliferation, indicating that ERK signaling only contributes to the tumor-promoting activity (invasion) of TGF-β1, rather than its antiproliferative activity in HCC. In addition, we suggest that TGF-β inhibits HCC cell proliferation through an ERK-independent pathway, such as through promoting the expression of cyclin-dependent inhibitors27.
Moreover, previous studies have shown that the ERK mediator AP-1 can interact with Smad, which forms an EMT-promoting Smad complex (EPSC) and thus mediates the expression of EMT-related genes28. Therefore, ERK and Smad signaling can function through independent and cross-talk mechanisms. In this study, we showed that treatment with TGF-β1 activated both ERK and Smad2/3 signaling and thus suggested that the ERK and Smad pathways might cooperatively induce EMT, which further promotes HCC cell invasion. Moreover, for the first time, molecular mechanism investigation revealed that TGF-β1 activated ERK1/2 signaling through the Smad2/3 pathway, but not the Slug pathway, in HCC cells, which further expands the understanding of the molecular mechanism underlying the activation of ERK1/2 by TGF-β1 in human cancers.
In conclusion, the present study demonstrates that TGF-β1 promotes HepG2 cell migration and invasion depending on the activation of ERK1/2 signaling, while showing TGF-β1’s suppressive effects on cell proliferation in an ERK-independent manner. Blocking of ERK1/2 signaling effectively impairs the promoting effects of TGF-β1 on HCC cell migration and invasion without affecting its antiproliferating function. These findings suggest that ERK1/2 signaling may become a potential therapeutic target for TGF-β1-responsive HCC.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- 3. Castellone MD, Laukkanen MO. TGF-beta1, WNT, and SHH signaling in tumor progression and in fibrotic diseases. Front Biosci (Schol Ed). 2017;9:31–45. [DOI] [PubMed] [Google Scholar]
- 4. Kubosch EJ, Heidt E, Bernstein A, Bottiger K, Schmal H. The Transwell coculture of human synovial mesenchymal stem cells with chondrocytes leads to self-organization, chondrogenic differentiation, and secretion of TGFbeta. Stem Cell Res Ther. 2016;7(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou HS, Su XF, Fu XL, Wu GZ, Luo KL, Fang Z, Yu F, Liu H, Hu HJ, Chen LS, Cai B, Tian ZQ. Mesenchymal stem cells promote pancreatic adenocarcinoma cells invasion by transforming growth factor-beta1 induced epithelial-mesenchymal transition. Oncotarget 2016;7(27):41294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Loboda A, Sobczak M, Jozkowicz A, Dulak J. TGF-beta1/Smads and miR-21 in renal fibrosis and inflammation. Mediators Inflamm. 2016;2016:8319283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dooley S, ten Dijke P. TGF-beta in progression of liver disease. Cell Tissue Res. 2012;347(1):245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heldin CH, Landstrom M, Moustakas A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr Opin Cell Biol. 2009;21(2):166–76. [DOI] [PubMed] [Google Scholar]
- 9. Seoane J. Escaping from the TGFbeta anti-proliferative control. Carcinogenesis 2006;27(11):2148–56. [DOI] [PubMed] [Google Scholar]
- 10. Lee MK, Pardoux C, Hall MC, Lee PS, Warburton D, Qing J, Smith SM, Derynck R. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 2007;26(17):3957–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fu H, Hu Z, Wen J, Wang K, Liu Y. TGF-beta promotes invasion and metastasis of gastric cancer cells by increasing fascin1 expression via ERK and JNK signal pathways. Acta Biochim Biophys Sin. (Shanghai) 2009;41(8):648–56. [DOI] [PubMed] [Google Scholar]
- 12. Zhou Y, Yang S, Zhang P. Effect of exogenous Fetuin-A on TGF-beta/Smad signaling in hepatic stellate cells. Biomed Res Int. 2016;2016:8462615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farid IM, Hamza IM, El-Abd DM, Mohyi AM, AbdulLatif MM, Aref AT, Hamza DM. Transforming growth factor-beta1 gene expression in hepatocellular carcinoma: A preliminary report. Arab J Gastroenterol. 2014;15(3–4):142–7. [DOI] [PubMed] [Google Scholar]
- 14. Pang M, Teng Y, Huang J, Yuan Y, Lin F, Xiong C. Substrate stiffness promotes latent TGF-beta1 activation in hepatocellular carcinoma. Biochem Biophys Res Commun. 2017;483(1):553–8. [DOI] [PubMed] [Google Scholar]
- 15. Ji F, Fu SJ, Shen SL, Zhang LJ, Cao QH, Li SQ, Peng BG, Liang LJ, Hua YP. The prognostic value of combined TGF-beta1 and ELF in hepatocellular carcinoma. BMC Cancer 2015;15:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen Z, Xie B, Zhu Q, Xia Q, Jiang S, Cao R, Shi L, Qi D, Li X, Cai L. FGFR4 and TGF-beta1 expression in hepatocellular carcinoma: Correlation with clinicopathological features and prognosis. Int J Med Sci. 2013;10(13):1868–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang M, Sui C, Dai B, Shen W, Lu J, Yang J. PEG10 is imperative for TGF-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Oncol Rep. 2017;37(1):510–8. [DOI] [PubMed] [Google Scholar]
- 18. Ito Y, Sasaki Y, Horimoto M, Wada S, Tanaka Y, Kasahara A, Ueki T, Hirano T, Yamamoto H, Fujimoto J, Okamoto E, Hayashi N, Hori M. Activation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology 1998;27(4):951–8. [DOI] [PubMed] [Google Scholar]
- 19. Xu MY, Chen R, Yu JX, Liu T, Qu Y, Lu LG. AZGP1 suppresses epithelial-to-mesenchymal transition and hepatic carcinogenesis by blocking TGFbeta1-ERK2 pathways. Cancer Lett. 2016;374(2):241–9. [DOI] [PubMed] [Google Scholar]
- 20. Dontula R, Dinasarapu A, Chetty C, Pannuru P, Herbert E, Ozer H, Lakka SS. MicroRNA 203 modulates glioma cell migration via Robo1/ERK/MMP-9 signaling. Genes Cancer 2013;4(7–8):285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ban K, Peng Z, Kozar RA. Inhibition of ERK1/2 worsens intestinal ischemia/reperfusion injury. PLoS One 2013;8(9):e76790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li B, Qiu T, Zhang P, Wang X, Yin Y, Li S. IKVAV regulates ERK1/2 and Akt signalling pathways in BMMSC population growth and proliferation. Cell Prolif. 2014;47(2):133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Luca A, Maiello MR, D’Alessio A, Pergameno M, Normanno N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: Role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets 2012;16(Suppl 2):S17–27. [DOI] [PubMed] [Google Scholar]
- 24. Fukawa T, Kajiya H, Ozeki S, Ikebe T, Okabe K. Reactive oxygen species stimulates epithelial mesenchymal transition in normal human epidermal keratinocytes via TGF-beta secretion. Exp Cell Res. 2012;318(15):1926–32. [DOI] [PubMed] [Google Scholar]
- 25. Xie L, Law BK, Chytil AM, Brown KA, Aakre ME, Moses HL. Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia 2004;6(5):603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boye A, Kan H, Wu C, Jiang Y, Yang X, He S, Yang Y. MAPK inhibitors differently modulate TGF-beta/Smad signaling in HepG2 cells. Tumour Biol. 2015;36(5):3643–51. [DOI] [PubMed] [Google Scholar]
- 27. Shimizu T, Yokomuro S, Mizuguchi Y, Kawahigashi Y, Arima Y, Taniai N, Mamada Y, Yoshida H, Akimaru K, Tajiri T. Effect of transforming growth factor-beta1 on human intrahepatic cholangiocarcinoma cell growth. World J Gastroenterol. 2006;12(39):6316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fuxe J, Vincent T, Garcia de Herreros A. Transcriptional crosstalk between TGF-beta and stem cell pathways in tumor cell invasion: Role of EMT promoting Smad complexes. Cell Cycle 2010;9(12):2363–74. [DOI] [PubMed] [Google Scholar]