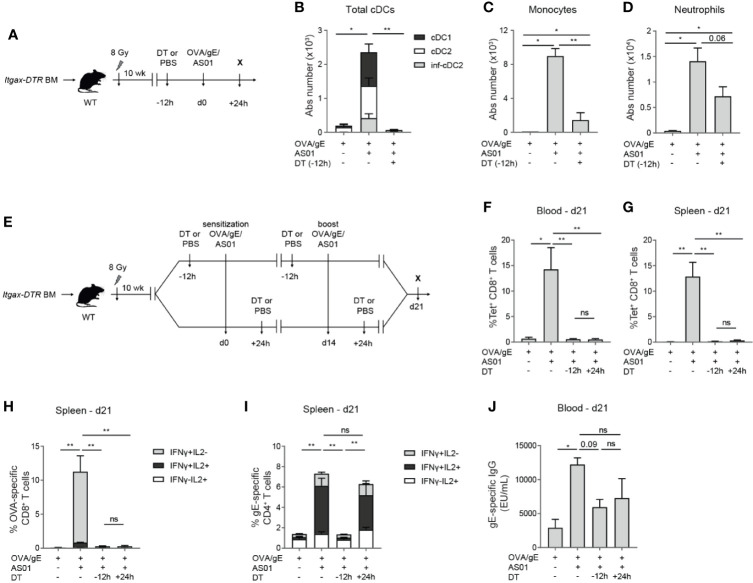
Figure 3.
Cellular and humoral adaptive immune responses depend on CD11c+ cells. (A) Schematic representation of Itgax-DTR BM chimeras. (B–D) Absolute number of distinct cDC subsets (B), monocytes (C), and neutrophils (D) in the dLN 24 h after i.m. immunization with OVA/gE/AS01 (0.5/2.5/2.5 µg) per injection site with or without prior DT-mediated depletion of CD11c+ cells. (E) Schematic representation of Itgax-DTR BM chimeras treated 12 h prior to or 24 h after OVA/gE/AS01 (0.5/2.5/2.5 µg) per injection site for both sensitization and challenge. (F, G) Percentage OVA257–264 MHCI-Tetramer+ CD8+ T cells in the blood (F) and spleen (G) of Itgax-DTR BM chimeric mice 21 days after i.m. immunization at d0 and challenge at d14 with OVA/gE/AS01 (0.5/2.5/2.5 µg) per injection site and DT treatment. (H) Percentage IFN-γ and/or IL2 producing CD8+ T cells upon OVA restimulation in the spleen of Itgax-DTR BM chimeric mice 21 days after i.m. immunization at d0 and challenge at d14 with OVA/gE/AS01 (0.5/2.5/2.5 µg) per injection site and DT treatment. (I) Percentage IFN-γ and/or IL2 producing CD4+ T cells upon VZV gE restimulation in the spleen of Itgax-DTR BM chimeric mice 21 days after i.m. immunization at d0 and challenge at d14 with OVA/gE/AS01 (0.5/2.5/2.5 µg) per injection site and DT treatment. (J) Total VZV gE specific IgG levels in the serum of Itgax-DTR BM chimeric mice 21 days after i.m. immunization at d0 and challenge at d14 with OVA/gE/AS01 (0.5/2.5/2.5 µg) per injection site and DT treatment. Data are representative of 4 independent experiments (n = 2–5 mice per group). Error bars indicate mean ± SEM (B–J). Data analyzed with a One-way ANOVA and Sidak’s multiple comparison test *p < 0.05, **p < 0.01, ns, non-significant.