Abstract
Background:
Epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) is a standard treatment in EGFR-mutated advanced non-small-cell lung cancer (NSCLC); however, previous data have suggested that EGFR-TKI has limited potential as adjuvant therapy. On the contrary, based on subset analysis with the immune checkpoint inhibitor (ICI) plus platinum-doublet chemotherapy in advanced NSCLC with EGFR mutation, we hypothesized that this combination was worth testing as adjuvant therapy in patients with EGFR-mutated NSCLC.
Methods:
Herein, we introduce our phase II study of cisplatin plus vinorelbine combined with atezolizumab as adjuvant therapy for completely resected NSCLC with EGFR mutation. Accrued patients will be pathological stage II–IIIA with completely resected NSCLC and whose tumors have EGFR mutation. Treatment comprises four cycles of cisplatin plus vinorelbine combined with atezolizumab followed by maintenance with atezolizumab. The primary endpoint is the disease-free survival (DFS) rate at 2 years. Secondary endpoints are DFS, overall survival, and safety. In total, 18 patients will be enrolled in this study.
Discussion:
Ongoing phase III trials of adjuvant ICI allow the inclusion of patients with EGFR mutation, but our current trial will provide the earliest clinical data on the efficacy of platinum-doublet chemotherapy with atezolizumab.
Keywords: adjuvant chemotherapy, atezolizumab, cisplatin, EGFR mutation, vinorelbine
Introduction
Epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) is the standard treatment in patients with EGFR-mutated advanced non-small-cell lung cancer (NSCLC).1 Recently, there has been increasing interest over the benefit of EGFR-TKI in patients with early stage NSCLC. Phase III trials compared EGFR-TKI as adjuvant therapy with platinum-doublet chemotherapy in patients with completely resected NSCLC with EGFR mutation.2 Although disease-free survival (DFS) in the EGFR-TKI group was significantly longer than that in the chemotherapy group, the overall survival (OS) showed no significant difference. Moreover, whether adjuvant EGFR-TKI therapy is effective in this population is controversial. Therefore, the current standard treatment in this population is still platinum-doublet chemotherapy, regardless of EGFR mutation status.
Immune-checkpoint inhibitors (ICIs), such as anti-programmed cell death 1 (PD-1) or programmed death ligand 1 (PD-L1) antibody, showed favorable antitumor activities for the treatment of advanced NSCLC.3 However, a meta-analysis, which compared anti-PD-1/PD-L1 antibody with docetaxel, reported that EGFR-mutated NSCLC was less likely to be sensitive to ICI than EGFR wild-type NSCLC.4 IMpower150 is a phase III trial that compared the combination of atezolizumab plus platinum-doublet chemotherapy plus bevacizumab with platinum-doublet chemotherapy plus bevacizumab in advanced nonsquamous NSCLC.5 In addition, subgroup analysis in patients with EGFR-mutated NSCLC demonstrated that platinum-doublet chemotherapy plus atezolizumab in EGFR-mutated NSCLC had longer progression-free survival (PFS) than chemotherapy [median PFS, 10.2 months versus 6.9 months; hazard ratio (HR), 0.61; 95% confidence interval, 0.36–1.03].6 Based on these backgrounds, we hypothesized that the combination of platinum-doublet chemotherapy and atezolizumab could potentially improve the cure rate in patients with completely resected NSCLC with EGFR mutation.
Study protocol
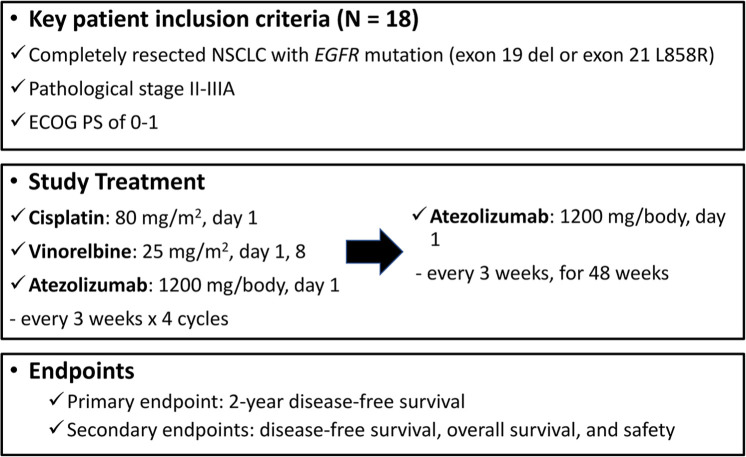
This study aims to investigate the efficacy of cisplatin plus vinorelbine combined with atezolizumab as adjuvant therapy for patients with completely resected NSCLC with EGFR mutation. It is a multicenter, single-arm prospective, phase II study. Figure 1 summarizes the study design.
Figure 1.
Study design.
ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor.
The primary endpoint is the DFS rate at 2 years. Secondary endpoints are DFS, OS, and safety.
Patients who fulfill all the inclusion criteria will be included, whereas those who meet the exclusion criteria will be excluded. The key inclusion criteria are: (a) patients who have complete resection within 21–56 days of registration; (b) patients with a pathologically confirmed tumor of stage II-IIIA NSCLC with EGFR mutation (exon 19 deletion or exon 21 Leu858Arg); (c) patients aged ⩾20 years; (d) patients with Eastern Cooperative Oncology Group performance status of 0 to 1; (e) patients with adequate organ function. The key exclusion criteria are: (a) any history of malignancy in the past 5 years; (b) any history of autoimmune disease (patients who have controlled thyroid dysfunction or skin disease that does not require systemic therapy are eligible); (c) interstitial lung disease based on chest computed tomography (CT); (d) positive for human immunodeficiency virus, hepatitis B virus antigen, or hepatitis C virus RNA; (e) history of active infection within 14 days of registration; (f) history of taking corticosteroid or other immunosuppressive drugs within 14 days of registration.
Treatment
Patients will be administered cisplatin (80 mg/m2 intravenously on day 1), vinorelbine (25 mg/m2 intravenously on days 1 and 8), and atezolizumab (1200 mg/body intravenously on day 1) every 3 weeks for four cycles. Thereafter, patients will be administered atezolizumab monotherapy (1200 mg/body intravenously every 3 weeks for 1 year), until relapse, or unacceptable toxicity.
Follow up and assessment
To assess efficacy, chest and abdominal CT will be performed every 6 months. Magnetic resonance imaging or CT of the brain will be performed at 2 years from registration. Adverse events will be graded using the Common Terminology Criteria for Adverse Events, Version 5.0.
Statistical analysis
In advanced NSCLC, platinum-doublet chemotherapy with atezolizumab that marked an HR of 0.37–0.94 in the PFS was compared with platinum-doublet chemotherapy. Thus, the sample size for this study was calculated based on the hypothesis that the HR of cisplatin plus vinorelbine combined with atezolizumab will achieve 55% improvement in DFS over cisplatin plus vinorelbine. A total of 16 eligible patients are required to ensure a statistical power of 0.80 at a one-sided α-error of 0.10. Assuming a drop-out rate of 12%, 18 patients need to be enrolled in this study.
Ethical considerations
The study will be conducted in compliance with the principles of the Declaration of Helsinki, and the Wakayama Medical University Institutional Review Board approved this protocol on 2 July 2019 (approval number: 1-01008A). This clinical trial was registered in the Japic Clinical Trials Information, Japan (protocol identification no. JapicCTI-194849). Written informed consent will be obtained from all patients before any screening or inclusion procedures.
Discussion
To develop novel adjuvant treatment among patients with EGFR-mutated NSCLC who underwent complete resection, EGFR-TKI was tested in clinical trials. Previously, first-generation EGFR-TKI as adjuvant therapy did not show prolongation of OS compared with platinum-doublet chemotherapy despite prolongation of DFS.2,7 The recent phase III trial compared osimertinib with placebo in patients with completely resected NSCLC with EGFR mutation and showed a significant prolongation of DFS compared with placebo (HR, 0.17).8 However, as their median follow up was just 22.1 months, it cannot be known if EGFR-TKI can induce cure in this population. Therefore, various strategies besides EGFR-TKI need to be considered in this population.
Among patients with metastatic NSCLC with EGFR mutation, subgroup analysis of the IMpower150 trial suggested the efficacy of adding ICIs to chemotherapy, whereas subgroup analysis of another phase III trial (IMpower130) did not show prolongation of PFS in the atezolizumab arm.6,9 As both were small sample-sized subgroup analyses, it is not possible to draw any definite conclusions. Furthermore, preclinical study justified the use of ICI in early stage NSCLC because completely resected tumors have a lower tumor burden and different microenvironment compared with the metastatic setting.10
The necessity for the addition of anti-angiogenic antibody is another debatable issue. Although the quadrat regimen (platinum-doublet chemotherapy + bevacizumab + atezolizumab) showed the most promising efficacy in metastatic NSCLC (IMpower 150 trial), the pivotal adjuvant phase III trial (E1505) failed to demonstrate the benefit of adding bevacizumab to platinum-doublet chemotherapy and it increased toxicity.11 Therefore, we are determined to avoid the addition of bevacizumab in our trial.
Conclusion
Ongoing phase III trials of adjuvant ICI allow the inclusion of patients with EGFR mutation, but our current trial will provide the earliest clinical data on the efficacy of platinum-doublet chemotherapy with atezolizumab. Thus, the current trial for completely resected NSCLC with EGFR mutation will provide a solution for an important clinical question.
Acknowledgments
We thank the patients, their families, and all investigators participating in the study.
Footnotes
Author contributions: RS, HA, and NY were involved in the study conception and design. RS, HA, KY, and NY will be involved in the analysis and interpretation of the data. All authors have read and approved the final manuscript.
Availability of data and material: All data generated or analyzed during this study are included in this published article.
Conflict of interest statement: Dr. Shibaki has nothing to disclose. Dr. Akamatsu reports personal fees from AstraZeneca K.K., personal fees from Boehringer Ingelheim Japan Inc., personal fees from Bristol-Myers Squibb, grants and personal fees from Chugai Pharmaceutical Co. Ltd., personal fees from Eli Lilly Japan K.K., grants and personal fees from MSD K.K., personal fees from Novartis Pharma K.K., personal fees from Ono Pharmaceutical Co. Ltd., personal fees from Taiho Pharmaceutical Co. Ltd., from null, outside the submitted work. Dr. Kato reports grants and personal fees from Abbvie, grants and personal fees from Amgen, grants and personal fees from AstraZeneca, grants and personal fees from Bristol Myers Squibb, grants and personal fees from Chugai, grants and personal fees from Eli Lilly, grants and personal fees from Merck Biopharma, grants and personal fees from MSD, grants and personal fees from Novartis, grants and personal fees from Ono, grants and personal fees from Pfizer, grants and personal fees from Taiho, personal fees from Boehringer Ingelheim, personal fees from Daiichi-Sankyo, personal fees from Nippon Kayaku, personal fees from Takeda, grants from Regeneron, outside the submitted work. Dr. Nishino reports personal fees from AstraZeneca, personal fees from Chugai pharmaceutical, grants and personal fees from Nippon Boehringer Ingelheim, personal fees from Eli Lilly Japan, personal fees from Roche Diagnostics, personal fees from Novartis, personal fees from Pfizer, personal fees from Ono Pharmaceutical, personal fees from Merk, outside the submitted work. Dr. Okada reports grants and personal fees from Chugai Pharmaceutical, during the conduct of the study. Dr. MItsudomi reports grants and personal fees from CHugai, during the conduct of the study; grants and personal fees from Boehringer Ingelheim, grants and personal fees from AstraZeneca, grants and personal fees from Pfizer, personal fees from Novartis, grants and personal fees from MSD, personal fees from BMS, personal fees from Ono, grants and personal fees from Taiho, grants and personal fees from Daiichi-Sankyo, personal fees from Janssen, personal fees from Eli-Lilly, outside the submitted work. Dr. Wakuda reports grants and personal fees from Chugai Pharmaceutical Co., Ltd., personal fees from Taiho Pharmaceutical, personal fees from Boehringer Ingelheim, personal fees from Eli Lilly K.K., personal fees from Ono Pharmaceutical, personal fees from MSD, grants and personal fees from Astrazeneca, grants from Novartis, grants from Abbvie, outside the submitted work. Dr. Yoshimura reports personal fees from Astra Zeneca, personal fees from Chugai Pharma, personal fees from Eli lilly, personal fees from Eizai, personal fees from Nihon-kayaku, personal fees from Taiho, personal fees from Takeda, outside the submitted work. Dr. Yamamoto reports grants and personal fees from MSD K.K., grants and personal fees from AstraZeneca, grants and personal fees from ONO PHARMACEUTICAL CO., LTD., personal fees from Thermo Fisher Scientific, grants and personal fees from DAIICHI SANKYO CO., LTD., grants and personal fees from TAIHO PHARMACEUTICAL CO., LTD., grants and personal fees from Takeda Pharmaceutical CO., LTD., grants and personal fees from Chugai Pharmaceutical CO., LTD., grants and personal fees from Eli Lilly Japan K.K., grants and personal fees from Boehringer-Ingelheim, grants and personal fees from Novartis, grants and personal fees from Pfizer Inc., personal fees from Bristol-Myers Squibb, personal fees from Life Technologies Japan Ltd., personal fees from NIPPON KAYAKU, personal fees from Merk Biopharma, grants from Astellas Pharma Inc., grants from TSUMURA & CO., grants from SHIONOGI Co., Ltd., grants from AbbVie GK., grants from Amgen Inc., grants from KYORIN Pharmaceutical Co., Ltd., grants from Eisai Co., Ltd., grants from TERUMO CORPORATION, grants from Toppan Printing Co., Ltd., grants from TOSOH, outside the submitted work. Dr. Nakagawa reports grants and personal fees from AstraZeneca K.K., grants and personal fees from Astellas Pharma Inc., grants and personal fees from MSD K.K., grants, personal fees and other from Ono Pharmaceutical Co.,Ltd., grants and personal fees from Nippon Boehringer Ingelheim Co.,Ltd., grants and personal fees from Novartis Pharma K.K., grants, personal fees and other from Pfizer Japan Inc., grants and personal fees from Bristol Myers Squibb Company, grants, personal fees and other from Eli Lilly Japan K.K., grants and personal fees from Chugai Pharmaceutical Co.,Ltd., grants and personal fees from Daiichi Sankyo Co., Ltd., grants and personal fees from Merck Serono Co., Ltd./ Merck Biopharma Co., Ltd., during the conduct of the study; personal fees from Clinical Trial Co., Ltd., personal fees from MEDICUS SHUPPAN,Publishers Co., Ltd., personal fees from Care Net, Inc, personal fees from Reno. Medical K.K., personal fees and other from KYORIN Pharmaceutical Co.,Ltd., personal fees from Medical Review Co., Ltd., personal fees from Roche Diagnostics K.K., personal fees from Bayer Yakuhin, Ltd, personal fees from Medical Mobile Communications co., Ltd, personal fees from 3H Clinical Trial Inc., personal fees from Nichi-Iko Pharmaceutical Co., Ltd., grants, personal fees and other from Takeda Pharmaceutical Co.,Ltd., grants and personal fees from Taiho Pharmaceutical Co.,Ltd., grants and personal fees from SymBio Pharmaceuticals Limited., personal fees from NANZANDO Co.,Ltd., personal fees from YODOSHA CO., LTD., personal fees from Nikkei Business Publications, Inc, personal fees from Thermo Fisher Scientific K.K., personal fees from YOMIURI TELECASTING CORPORATION., personal fees from Nippon Kayaku Co.,Ltd., grants and personal fees from AbbVie Inc, grants from inVentiv Health Japan, grants from ICON Japan K.K., grants from GRITSONE ONCOLOGY.INC, grants from PAREXEL International Corp., grants from Kissei Pharmaceutical Co.,Ltd., grants from EPS Corporation., grants from Syneos Health., grants from Pfizer R&D Japan G.K., grants from A2 Healthcare Corp., grants from Quintiles Inc. / IQVIA Services JAPAN K.K., grants from EP-CRSU CO., LTD., grants from Linical Co.,Ltd., grants from Eisai Co., Ltd., grants from CMIC Shift Zero K.K., grants from Kyowa Hakko Kirin Co.,Ltd, grants from Bayer Yakuhin, Ltd, grants from EPS International Co.,Ltd,., grants from Otsuka Pharmaceutical Co., Ltd., outside the submitted work.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is conducted by the West Japan Oncology Group (WJOG) under a funding contract with Chugai Pharmaceutical Co. Ltd, Tokyo, Japan.
ORCID iD: Hiroaki Akamatsu  https://orcid.org/0000-0001-5856-5512
https://orcid.org/0000-0001-5856-5512
Contributor Information
Ryota Shibaki, Internal Medicine III, Wakayama Medical University, Wakayama, Japan.
Hiroaki Akamatsu, Internal Medicine III, Wakayama Medical University, 811-1, Kimiidera, Wakayama City, Wakayama 641-8509, Japan.
Terufumi Kato, Deparment of Thoracic Oncology Group, Kanagawa Cancer Center, Yokohama, Japan.
Kazumi Nishino, Department of Thoracic Oncology, Osaka International Cancer Institute, Osaka, Japan.
Morihito Okada, Department of Surgical Oncology, Research Center for Radiation Casualty Medicine, Hiroshima University, Hiroshima, Japan.
Tetsuya Mitsudomi, Department of Surgery, Kindai University Hospital, Osaka, Japan.
Kazushige Wakuda, Division of Thoracic Oncology, Shizuoka Cancer Center, Shizuoka, Japan.
Kenichi Yoshimura, Medical Center for Translational and Clinical Research, Hiroshima University, Hiroshima, Japan.
Nobuyuki Yamamoto, Internal Medicine III, Wakayama Medical University, Wakayama, Japan.
Kazuhiko Nakagawa, Department of Medical Oncology, Kindai University Faculty of Medicine, Osaka, Japan.
References
- 1. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018; 378: 113–125. [DOI] [PubMed] [Google Scholar]
- 2. Zhong W-Z, Wang Q, Mao W-M, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II–IIIA (N1–N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol 2018; 19: 139–148. [DOI] [PubMed] [Google Scholar]
- 3. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang Q, Zhang H, Hai J, et al. Impact of PD-L1 expression, driver mutations and clinical characteristics on survival after anti-PD-1/PD-L1 immunotherapy versus chemotherapy in non-small-cell lung cancer: a meta-analysis of randomized trials. Oncoimmunology 2018; 7: e1396403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378: 2288–2301. [DOI] [PubMed] [Google Scholar]
- 6. Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019; 7: 387–401. [DOI] [PubMed] [Google Scholar]
- 7. Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): a randomized, double-blind, phase III trial. J Clin Oncol 2015; 33: 4007–4014. [DOI] [PubMed] [Google Scholar]
- 8. Wu YL, Tsuboi M, He J, et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med 2020; 383: 1711–1723. [DOI] [PubMed] [Google Scholar]
- 9. West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20: 924–937. [DOI] [PubMed] [Google Scholar]
- 10. Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017; 545: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wakelee HA, Dahlberg SE, Keller SM, et al. Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 2017; 18: 1610–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]