Figure 3.
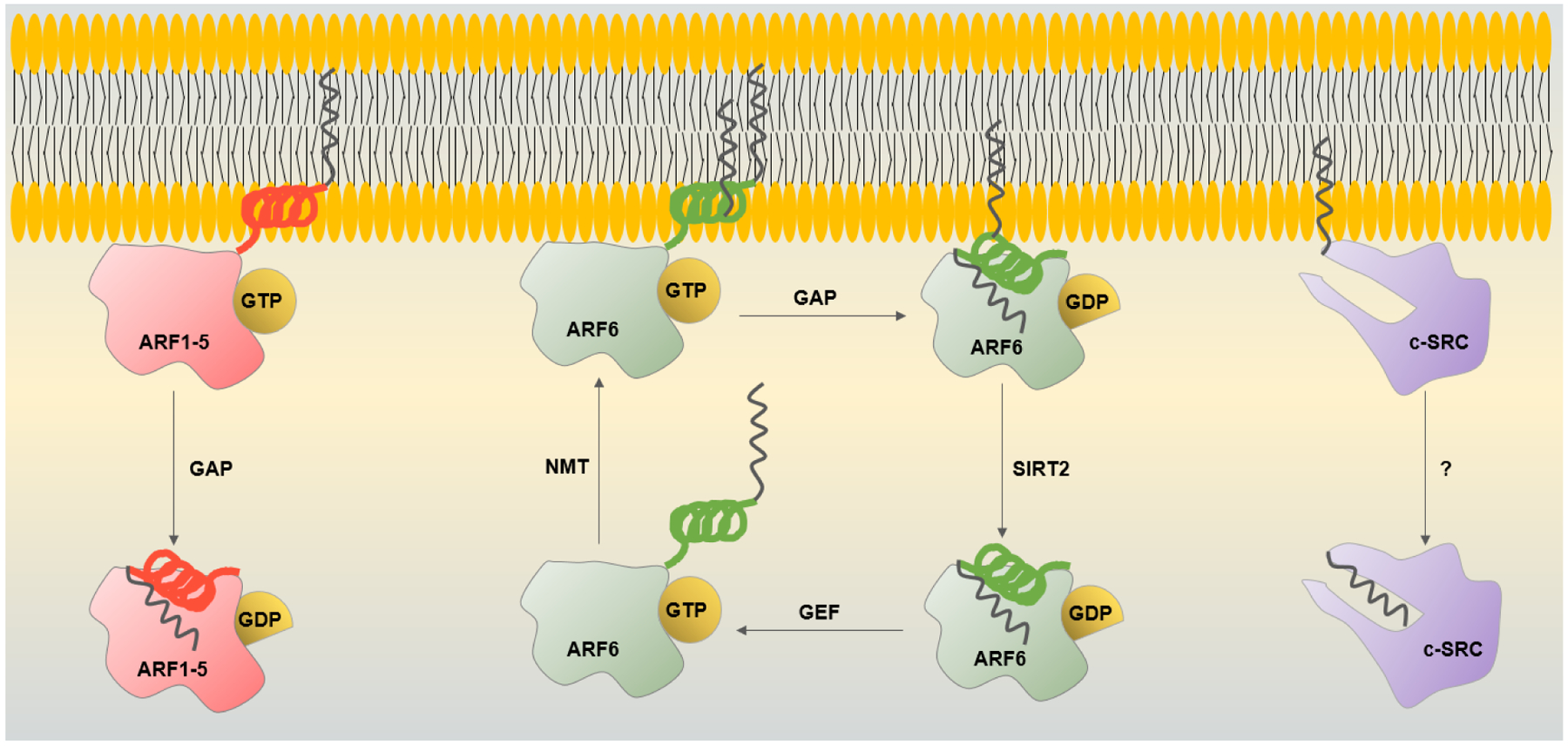
Myristoyl switches of ARF GTPases and c-Src. ARF1–5 are membrane-bound in the GTP-bound state via their amphipathic helix with one myristoyl group. The GTP hydrolysis facilitated by GAP causes a conformational change that sequesters the myristoylated helix releasing ARF1–5 from membranes. ARF6 has an additional myristoyl chain on lysine 3 that helps to retain ARF6-GDP on the plasma membrane and endomembranes. SIRT2 removes lysine myristoylation allowing ARF6-GDP to be activated by GEF. NMT myristoylates lysine 3 of ARF6-GTP, promoting its plasma membrane localization and completing the cycle. c-Src contains a hydrophobic pocket in its SH3 domain, that sequesters the myristoyl chain, but what causes the switch is unclear.
