Scheme 1. General Synthetic Routes for the Synthesis of Compounds 1–21.
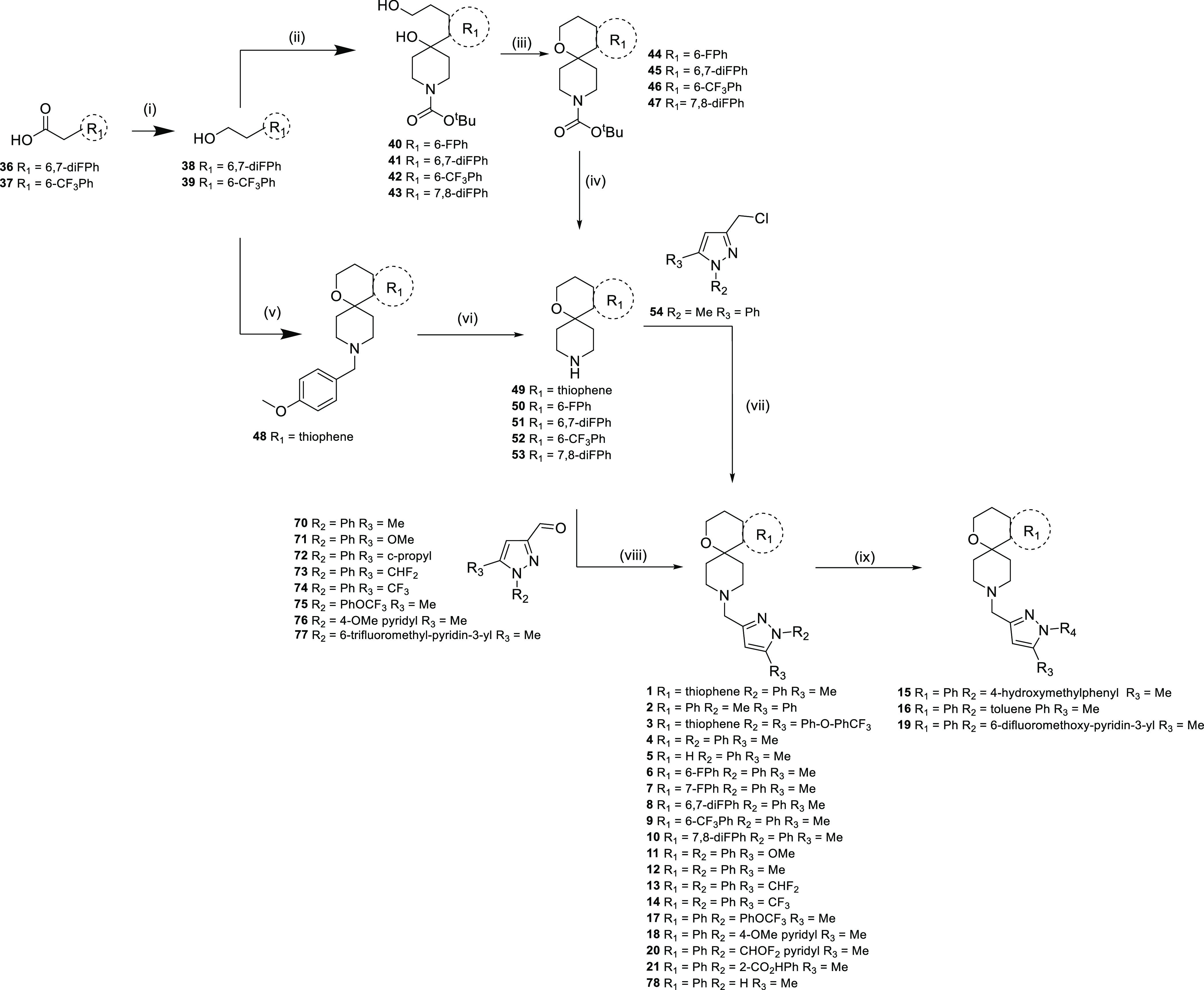
Reagents and conditions: (i) BH3.THF, THF, 100 °C, and 1 h; (ii) nBuLi, THF, −78 °C, 1 h, then tert-butyl 4-oxopiperidine-1-carboxylate −78 °C—rt, and 18 h; (iii) MeSO2Cl, Et3N, DCM, reflux, and 1.5 h; (iv) TFA, DCM, and 18 h; (v) 1-[(4-methoxyphenyl)methyl]piperidin-4-one, MeSO3H, PhMe, reflux, Dean–Stark, and 18 h; (vi) 1-chloroethyl carbonochloridate, DCM, 0 °C then MeOH, reflux, and 1 h; (vii) DIPEA, DMSO, rt, and 18 h; (viii) AcOH or EtOH, reflux, and 2 h; (ix) R2B(OH)2, Cu(OAc)2, pyridine, DCM, and 2 h; (x) Et2O, rt, 1 h, then NaOMe, MeOH, rt, 18 h, then H2SO4, MeOH, reflux, and 48 h; (xi) PPh3, DIAD, THF, MeOH, rt, and 18 h; (xii) DIBAL, DCM, −78 °C—rt, then MnO2, DCM, rt, and 48 h; (xiii) AcOH, DCM, then NaBH(OAc)3, and 18 h; (xiv) R4B(OH)2, Cu(OAc)2, pyridine, DCM, rt, and 3–18 h.
