Abstract
According to Article 12 of Regulation (EC) No 396/2005, EFSA has reviewed the maximum residue levels (MRLs) currently established at European level for the pesticide active substance sulfuryl fluoride. To assess the occurrence of sulfuryl fluoride and fluoride ion residues in plants, processed commodities and livestock, EFSA considered the conclusions derived in the framework of Directive 91/414/EEC, the MRLs established by the Codex Alimentarius Commission as well as the European authorisations reported by Member States (including the supporting residues data). Based on the assessment of the available data, MRLs were calculated, but a consumer risk assessment could be carried out for sulfuryl fluoride only. Although no apparent risk to consumers was identified for sulfuryl fluoride, a standard consumer risk assessment to fluoride ion could not be performed, lacking information on the toxicological reference values for fluoride. Hence, an ‘overall’ consumer risk assessment could not be performed, only tentative MRLs proposal could be derived and measures for reduction of the consumer exposure should also be considered. Nevertheless, considering that fluoride ion is naturally occurring in food of plant and animal origin, EFSA performed an indicative calculation of the consumer exposure to estimate whether the uses currently authorised will contribute significantly to the overall consumer exposure to fluoride.
Keywords: sulfuryl fluoride, MRL review, Regulation (EC) No 396/2005, consumer risk assessment, insecticide, fumigant, fluoride ion
Summary
Sulfuryl fluoride was included in Annex I to Directive 91/414/EEC on 1 November 2010 by Commission Directive 2010/38/EU, and has been deemed to be approved under Regulation (EC) No 1107/2009, in accordance with Commission Implementing Regulation (EU) No 540/2011, as amended by Commission Implementing Regulation (EU) No 541/2011.
As the active substance was approved after the entry into force of Regulation (EC) No 396/2005 on 2 September 2008, the European Food Safety Authority (EFSA) is required to provide a reasoned opinion on the review of the existing maximum residue levels (MRLs) for that active substance in compliance with Article 12(1) of the aforementioned regulation.
As the basis for the MRL review, on 18 March 2019, EFSA initiated the collection of data for this active substance. In a first step, Member States and the United Kingdom were invited to submit by 17 April 2019 their national Good Agricultural Practices (GAPs) in a standardised way, in the format of specific GAP forms, allowing the designated rapporteur Member State, Austria, to identify the critical GAPs in the format of a specific GAP overview file. Subsequently, Member States were requested to provide residue data supporting the critical GAPs, within a period of 1 month, by 8 September 2019. On the basis of all the data submitted by Member States, the United Kingdom and by the European Union Reference Laboratories for Pesticides Residues (EURLs), EFSA asked the rapporteur Member State (RMS) to complete the Pesticide Residues Overview File (PROFile) and to prepare a supporting evaluation report. The PROFile and evaluation report, together with Pesticide Residues Intake Model (PRIMo) calculations and an updated GAP overview file were provided by the RMS to EFSA on 12 December 2019. Subsequently, EFSA performed the completeness check of these documents with the RMS. The outcome of this exercise including the clarifications provided by the RMS, if any, has been compiled in the completeness check report.
Based on the information provided by the RMS, Member States, the United Kingdom and the EURLs, and taking into account the conclusions derived by EFSA in the framework of Directive 91/414/EEC and the MRLs established by the Codex Alimentarius Commission, EFSA prepared in May 2020 a draft reasoned opinion, which was circulated to Member States and EURLs for consultation via a written procedure. All comments received by 22 June 2020 were evaluated by EFSA. As comments received triggered significant modifications of the assessment, the revised reasoned opinion was further circulated for a second commenting via a written procedure. The additional comments received by 27 November 2020 were evaluated by EFSA and considered during the finalisation of the reasoned opinion. The following conclusions are derived.
The nature of sulfuryl fluoride in plant commodities was not investigated in metabolism studies. Instead, information from public literature on the mode of degradation of sulfuryl fluoride in several food commodities was available. However, this information was considered not sufficient to fully elucidate the nature of the residues expected in food matrices upon fumigation. Tentative residue definitions for enforcement and risk assessment were proposed, i.e. sulfuryl fluoride and fluoride ion expressed individually. Depending on the results of the additional studies elucidating the nature of residues in unprocessed products, further information might be required also for processed products (investigation of hydrolytic stability of metabolites identified in treated products); hence, the residue definitions for processed products are also set on a tentative basis. Since sulfuryl fluoride is authorised only as fumigation on the interior of buildings, studies on rotational crops are not relevant.
Fully validated analytical methods are available for the enforcement of the proposed residue definitions in high oil and dry matrices but also in dried fruits at the limits of quantification (LOQs) of 0.004–0.01 mg/kg for sulfuryl fluoride and 0.05–5 mg/kg for fluoride ion. Analytical methods for the enforcement of fluoride ion in acidic and high‐water content commodities, in tea, coffee beans, herbal infusions, carobs, hops and spices are not available. According to the EURLs, an LOQ of 0.005 mg/kg for sulfuryl fluoride is achievable in high water, high oil, dry matrices and tea by using a single residue method. An analytical method for the enforcement of fluoride ion is currently not available to the EURLs. An analytical method for the enforcement of the proposed residue definitions in cocoa beans is not available and is still required.
The data from the available residue trials are considered sufficient to calculate MRL and risk assessment values for the first residue definition (i.e. sulfuryl fluoride) for all commodities under evaluation.
As regards the second residue definition (fluoride ion), EFSA considered the results of the trials and the information on the background levels of fluoride naturally occurring in plant commodities. Based on the available data, it was possible to calculate MRL and risk assessment values for all plant commodities, except for dry pulses, oilseeds, oil fruits, coffee beans, carobs, hops, spices and sugar plants for which no information on the background levels nor residue trials were available.
EFSA calculated MRL recommendations for raisins and cereals milling products for further risk management considerations, noting that according to Regulation (EC) No 396/2005, the setting of MRLs is not foreseen for processed products.
Considering the data gaps related to the nature of residues in the treated crops and to sufficiently validated analytical methods for enforcement in cocoa beans, in acidic and high‐water content commodities, in tea and in herbal infusions, the missing residue trials on cereals and raisins and pending more detailed information on the residue study on cocoa beans and on the background levels, all calculated MRLs should be considered tentative only.
Sulfuryl fluoride is authorised for use on cereals that might be fed to livestock. Nevertheless, considering that the authorised use is on empty stores and mills and the risk mitigation currently in place, livestock are not expected to be exposed to residues of sulfuryl fluoride and fluoride ion above the LOQ or above the background levels. Therefore, there is no need to further investigate residues in livestock. Regarding background concentrations from other sources than the authorised uses, data from the scientific opinion of the Panel on Dietetic Products, Nutrition and Allergies on fluoride were considered to derive MRLs for animal commodities. Nevertheless, these MRLs should be considered tentative only and should be confirmed by more detailed information on the background levels. Moreover, an analytical method for the enforcement of fluoride ion in animal commodities is not available.
Chronic and acute exposure calculations for all crops reported in the framework of this review were performed using revision 3.1 of the EFSA PRIMo for both sulfuryl fluoride and fluoride ion.
The exposure values calculated were compared with the toxicological reference values for sulfuryl fluoride, derived by EFSA. The highest chronic exposure was calculated for IE adult, representing 4% of the acceptable daily intake (ADI) and the highest acute exposure was calculated for pistachios, representing 4% of the acute reference dose (ARfD). Apart for the MRLs evaluated in the framework of this review, internationally recommended codex maximum residue limits (CXLs) have also been established for sulfuryl fluoride. Additional calculations of the consumer exposure, considering these CXLs, were therefore carried out. The highest chronic exposure represented 4% of the ADI (IE adults) and the highest acute exposure amounted to 5% of the ARfD (coconuts).
For fluoride ion, a standard consumer risk assessment could not be performed, lacking information on the toxicological reference values. Nevertheless, considering that fluoride ion is naturally occurring in food of plant and animal origin, EFSA performed an indicative calculation of the consumer exposure to estimate whether the uses currently authorised will contribute significantly to the overall consumer exposure to fluoride ion. According to these indicative calculations, the contribution from the authorised uses and CXLs to the overall fluoride exposure is low (except for the uses on cereals assessed by the JMPR). Nevertheless, an ‘overall’ conclusive consumer exposure assessment for the uses under consideration could not be performed, pending additional information on the toxicological profile of fluoride ion and comprehensive data on background levels for fluoride ion in plant and animal products.
Background
Regulation (EC) No 396/20051 (hereinafter also referred to as ‘the MRL Regulation’) establishes the rules governing the setting and the review of pesticide maximum residue levels (MRLs) at European level. Article 12(1) of that Regulation stipulates that the European Food Safety Authority (EFSA) shall provide, within 12 months from the date of the inclusion or non‐inclusion of an active substance in Annex I to Directive 91/414/EEC2 a reasoned opinion on the review of the existing MRLs for that active substance.
Sulfuryl fluoride was included in Annex I to Council Directive 91/414/EEC on 01 November 2010 by means of Commission Directive 2010/38/EU3 which has been deemed to be approved under Regulation (EC) No 1107/20094, in accordance with Commission Implementing Regulation (EU) No 540/20115, as amended by Commission Implementing Regulation (EU) No 541/20116. Therefore, EFSA initiated the review of all existing MRLs for that active substance.
Sulfuryl fluoride was evaluated in the framework of Directive 91/414/EEC by the United Kingdom, designated as rapporteur Member State (RMS). Subsequently, a peer review on the initial evaluation of the RMS was conducted by EFSA, leading to the conclusions as set out in the EFSA scientific output (EFSA, 2010). The approval of sulfuryl fluoride was restricted to uses as insecticide/nematicide (fumigant) applied by professional users in sealable structures, pending the submission of confirmatory information on the fate of sulfuryl fluoride in the atmosphere and for the residues of fluoride ion in milling products present in the machinery during the fumigation. Following submission and assessment of the confirmatory data (EFSA, 2015), the European Commission concluded that the data were insufficient to exclude that residue levels in milled products will not exceed the natural background levels for fluoride ion or will not meet the relevant maximum residue levels except by imposing further restrictions. The conditions of approval for sulfuryl fluoride were therefore amended by Commission Implementing Regulation (EC) No 270/20177, as following:
Only uses as insecticide/nematicide (fumigant) applied by professional users in sealable structures may be authorised insofar:
-
a)
these structures are empty; or
-
b)
where food or feed commodities are present in a fumigated facility, the users and the food business operators ensure that only the food or feed commodities compliant with the existing maximum residue levels for sulfuryl fluoride and fluoride ion set by Regulation (EC) No 396/2005 of the European Parliament and of the Council may enter the food and feed chain ; to this purpose, the users and the food business operators shall fully implement measures equivalent to the HACCP principles as laid down in Article 5 of Regulation (EC) No 852/2004 of the European Parliament and of the Council; in particular, the users shall identify the critical control point at which control is essential to prevent maximum residue levels to be exceeded, and establish and implement effective monitoring procedures at that critical control point.
According to the legal provisions, EFSA shall base its reasoned opinion in particular on the relevant assessment report prepared under Directive 91/414/EEC repealed by Regulation (EC) No 1107/2009. It should be noted, however, that, in the framework of Regulation (EC) No 1107/2009, only a few representative uses are evaluated, whereas MRLs set out in Regulation (EC) No 396/2005 should accommodate all uses authorised within the European Union (EU) and uses authorised in third countries that have a significant impact on international trade. The information included in the assessment report prepared under Regulation (EC) No 1107/2009 is therefore insufficient for the assessment of all existing MRLs for a given active substance.
To gain an overview of the pesticide residues data that have been considered for the setting of the existing MRLs, EFSA developed the Pesticide Residues Overview File (PROFile). The PROFile is an inventory of all pesticide residues data relevant to the risk assessment and MRL setting for a given active substance. This includes data on:
the nature and magnitude of residues in primary crops;
the nature and magnitude of residues in processed commodities;
the nature and magnitude of residues in rotational crops;
the nature and magnitude of residues in livestock commodities;
the analytical methods for enforcement of the proposed MRLs.
As the basis for the MRL review, on 18 March 2019, EFSA initiated the collection of data for this active substance. In a first step, Member States and the United Kingdom were invited to submit by 17 April 2019 their Good Agricultural Practices (GAPs) that are authorised nationally, in a standardised way, in the format of specific GAP forms. In the framework of this consultation, 15 Member States provided feedback on their national authorisations of sulfuryl fluoride. Based on the GAP data submitted, the designated RMS, Austria, was asked to identify the critical GAPs to be further considered in the assessment, in the format of a specific GAP overview file. Subsequently, in a second step, Member States were requested to provide residue data supporting the critical GAPs by 8 September 2019.
On the basis of all the data submitted by Member States, by the United Kingdom8 and the EU Reference Laboratories for Pesticides Residues (EURLs), EFSA asked RMS, Austria, to complete the PROFile and to prepare a supporting evaluation report. The PROFile and the supporting evaluation report, together with the Pesticide Residues Intake Model (PRIMo) calculations, were submitted to EFSA on 12 December 2019. Subsequently, EFSA performed the completeness check of these documents with the RMS. The outcome of this exercise including the clarifications provided by the RMS, if any, was compiled in the completeness check report.
Considering all the available information, and taking into account the MRLs established by the Codex Alimentarius Commission (CAC) (i.e. codex maximum residue limit; CXLs), EFSA prepared in May 2020 a draft reasoned opinion, which was circulated to Member States and EURLs for commenting via a written procedure. All comments received by 22 June 2020 were evaluated by EFSA. As comments received triggered significant modifications of the assessment, the revised reasoned opinion was further circulated for a second commenting via a written procedure. The additional comments received by 27 November 2020 were evaluated by EFSA and considered during the finalisation of the reasoned opinion.
The evaluation report submitted by the RMS (Austria, 2019), taking into account also the information provided by Member States during the collection of data, and the EURLs report on analytical methods (EURLs, 2019) are considered as main supporting documents to this reasoned opinion and, thus, made publicly available.
In addition, further supporting documents to this reasoned opinion are the completeness check report (EFSA, 2020a) and the Member States consultation reports (EFSA, 2020b,c). These reports are developed to address all issues raised in the course of the review, from the initial completeness check to the reasoned opinion. Furthermore, the exposure calculations for all crops reported in the framework of this review performed using the EFSA Pesticide Residues Intake Model (PRIMo) and the PROFiles as well as the GAP overview file listing all authorised uses are key supporting documents and made publicly available as background documents to this reasoned opinion. Screenshots of the report sheet of the PRIMo files are presented in Appendix C.
Terms of Reference
According to Article 12 of Regulation (EC) No 396/2005, EFSA shall provide a reasoned opinion on:
the inclusion of the active substance in Annex IV to the Regulation, when appropriate;
the necessity of setting new MRLs for the active substance or deleting/modifying existing MRLs set out in Annex II or III of the Regulation;
the inclusion of the recommended MRLs in Annex II or III to the Regulation;
the setting of specific processing factors as referred to in Article 20(2) of the Regulation.
The active substance and its use pattern
Sulfuryl fluoride (IUPAC) is considered by the International Organization for Standardization not to require a common name. Thus, there is no ISO common name for sulfuryl fluoride.
The chemical structures of the active substance and its main metabolite fluoride ion are reported in Appendix F.
The EU MRLs for sulfuryl fluoride and fluoride ion are established in Annexes IIIA of Regulation (EC) No 396/2005. Codex maximum residue limits (CXLs) for sulfuryl fluoride were also established by the Codex Alimentarius Commission (CAC). An application for the modification of the existing MRLs for sulfuryl fluoride and fluoride ion in chestnuts was assessed by EFSA in the framework of Article 10 of Regulation 396/2005 (EFSA, 2012). As EFSA could not conclude on the risk assessment related to the new use, the existing MRLs were not modified. For the purpose of this MRL review, all the uses of sulfuryl fluoride currently authorised within the EU as submitted by the Member States during the GAP collection, have been reported by the RMS in the GAP overview file. The critical GAPs identified in the GAP overview file were then summarised in the PROFiles for sulfuryl fluoride and fluoride ion and considered in the assessment. The details of the authorised critical GAPs for sulfuryl fluoride are given in Appendix A. The RMS did not report any use authorised in third countries that might have a significant impact on international trade.
Assessment
EFSA has based its assessment on the following documents:
the PROFiles submitted by the RMS;
the evaluation report accompanying the PROFiles (Austria, 2019);
the draft assessment report (DAR) and its addenda prepared under Council Directive 91/414/EEC (United Kingdom, 2004, 2009, 2015);
the conclusion on the peer review of the pesticide risk assessment of the active substance sulfuryl fluoride (EFSA, 2010);
the outcome of the consultation with Member States, the applicant and EFSA on the pesticide risk assessment for sulfuryl fluoride in light of confirmatory data (EFSA, 2015);
the review report on sulfuryl fluoride (European Commission, 2016);
the Joint Meeting on Pesticide residues (JMPR) Evaluation report (FAO, 2005);
the previous reasoned opinion on sulfuryl fluoride and fluoride ion (EFSA, 2012);
the scientific opinions of the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (EFSA, 2005, 2013).
The assessment is performed in accordance with the legal provisions of the uniform principles for evaluation and authorisation of plant protection products as set out in Commission Regulation (EU) No 546/20119 and the currently applicable guidance documents relevant for the consumer risk assessment of pesticide residues (European Commission, 1997a, 1997b, 1997c, 1997d, 1997e, 1997f, 1997g, 2000, 2010a, 2010b, 2017; OECD, 2011, 2013).
More detailed information on the available data and on the conclusions derived by EFSA can be retrieved from the list of end points reported in Appendix B.
1. Residues in plants
1.1. Nature of residues and methods of analysis in plants
1.1.1. Nature of residues in primary crops
Studies investigating the nature of residues in primary crops were not available during the peer review (United Kingdom, 2004, 2009; EFSA, 2010). Instead, the mode of degradation of sulfuryl fluoride in food commodities was investigated on the basis of public literature. According to the findings published in research papers, fluorine can replace hydrogen in many biologically active molecules, including amino acids, due to its small steric size. The experts of the peer review meeting (PRAPeR 05) considered the available information was not sufficient to fully elucidate the involved reactions; they concluded that a study to further investigate the nature of the residues expected in food matrices upon fumigation with sulfuryl fluoride is required. If fluoride is bound to proteins, an assessment should be performed whether the modified proteins are relevant for consumer safety and are to be taken into account in the residue definitions (EFSA, 2010).
No new metabolism studies were submitted under the current review. However, in the framework of this MRL review, an additional study was submitted, comparing the amount of total fluoride (free and bound fluoride) to free fluoride ion in treated cereal samples. According to this study, the total fluoride was found to be 26% higher compared to the free ion indicating a significant presence of bound fluoride in plant tissues (Austria, 2019). This study supports the assumption that fluorinated natural plant constituents may be formed. However, the study is not sufficient to address the data gap identified during the peer review, since no information is available on the nature of bound fluorine.
The data gap identified by the peer review is still not sufficiently addressed: the investigation of the nature of sulfuryl fluoride in all crops following the fumigation treatment has to be elucidated; a data gap is set to investigate whether metabolites/fluorinated natural food constituents are formed in food matrices following fumigation with sulfuryl fluoride.
1.1.2. Nature of residues in rotational crops
Sulfuryl fluoride is authorised only for fumigation of the interior of buildings. Therefore, studies investigating the nature of sulfuryl fluoride on rotational crops are not relevant.
1.1.3. Nature of residues in processed commodities
Currently, no studies are available investigating the nature of residues in processed products resulting from the use of sulfuryl fluoride according to the uses described in Appendix A.
Significant residues of parent sulfuryl fluoride were identified in tree nuts which would trigger the need to provide processing studies. However, due to the low dietary exposure to parent compound (see Section 3) standard hydrolysis studies for sulfuryl fluoride are considered not necessary.
Hydrolysis studies are not considered relevant for the fluoride ion, due to its nature.
It is noted that the nature of residues in unprocessed products is not yet fully elucidated and further information on the possible formation of reaction products such as fluorinated naturally occurring food constituents which might be of relevance for dietary risk assessment needs to be provided. Depending on the results of these studies, further data on the nature of residues in processed products might be required.
1.1.4. Methods of analysis in plants
During the peer review, separate analytical methods were available for the determination of sulfuryl fluoride and fluoride ion (EFSA, 2010).
In particular, for sulfuryl fluoride, a hyphenated analytical method based on GC coupled to ECD detector was validated in high oil content, dry matrices and dried fruits with limits of quantification (LOQs) of 0.004–0.01 mg/kg. The specific LOQs of sulfuryl fluoride per commodity are: 0.004 mg/kg in dried fruits and tree nuts; 0.01 mg/kg in cereals (United Kingdom, 2004, 2009). This primary method is supported by an independent laboratory validation (ILV). During the completeness check, the EURLs provided a single‐residue analytical method using Headspace‐GC/MS, for the routine analysis of sulfuryl fluoride with LOQs of 0.005 mg/kg in cucumbers (high water content), and 0.01 mg/kg in dry peas (dry commodities), almonds (high oil content commodities) and tea (difficult matrices) (EURLs, 2019).
For fluoride ion, an analytical method based on a fluoride ion specific electrode was validated in similar matrices with LOQs of 0.05–5 mg/kg. The specific LOQs of fluoride ion per commodity are: 0.5 mg/kg in wheat and maize grain, wheat flour, maize oil; 2.4 mg/kg in raisins, almonds, pecans, walnut; 2.5 mg/kg in figs, dates and pistachios; 2.6 mg/kg in prunes; 0.01 mg/kg in corn, barley, oats, rice and wheat (United Kingdom, 2004, 2009). This primary method is supported by an ILV. Analytical methods for the enforcement of fluoride ion in acidic and high‐water content commodities, in tea, coffee beans, herbal infusions, carobs, hops and spices are not available.
A fully validated analytical method for the enforcement of fluoride ion is currently not available to the EURLs (EURLs, 2019).
An analytical method for the enforcement of sulfuryl fluoride and fluoride ion in cocoa beans is not available and is still required.
1.1.5. Stability of residues in plants
The storage stability of sulfuryl fluoride in primary crops was neither investigated in the peer review (EFSA, 2010) nor new data were available under the current assessment, while the storage stability of fluoride residues has been demonstrated in wheat grain and flour, raisins, walnuts, maize grain and meal for at least 1 month at ambient temperatures and for at least 3 months at –20°C (EFSA, 2010).
Additional storage stability data for sulfuryl fluoride are not required; the residue trial samples were either analysed in the same day or within 1‐month storage at –18°C.
1.1.6. Proposed residue definitions
No metabolism studies are available, and a study is still required to elucidate the nature of the residues expected in food matrices upon fumigation with sulfuryl fluoride (see Section 1.1.1). Therefore, only tentative residue definitions for enforcement and risk assessment are proposed as sulfuryl fluoride and fluoride ion expressed individually.
Depending on the results of additional studies that should elucidate the nature of residues in unprocessed products, further information might be required also for processed products (investigation of hydrolytic stability of metabolites identified in treated products); hence, the residue definitions for processed products are also set on a tentative basis.
Two analytical methods for the enforcement of the proposed residue definitions at LOQs of 0.004–0.01 mg/kg for sulfuryl fluoride and 0.05–5 mg/kg for fluoride ion in several matrices covering the groups of high oil and dry matrices but also in dried fruits are available (EFSA, 2010). Analytical methods for the enforcement of fluoride ion in acidic and high‐water content commodities, in tea, coffee beans, herbal infusions, carobs, hops and spices are not available.
An analytical method for the enforcement of the proposed residue definitions in cocoa beans is not available and is still required.
According to the EURLs, an analytical method for the enforcement of sulfuryl fluoride at the LOQ of 0.005 mg/kg in high water, high oil, dry matrices and tea is available. An analytical method for the enforcement of fluoride ion is currently not available to the EURLs (EURLs, 2019).
1.2. Magnitude of residues in plants
1.2.1. Magnitude of residues in primary crops resulting from the authorised uses
To assess the magnitude of sulfuryl fluoride and fluoride ion residues resulting from the reported GAPs, EFSA considered all residue trials reported by the RMS in its evaluation report (Austria, 2019), as well as the residue trials evaluated in the framework of the peer review (United Kingdom, 2004, 2009; EFSA, 2010). Trials reported in the re‐registration report prepared by Belgium were also considered (Belgium, 2013).
All residue trial samples considered in this framework were stored in compliance with the conditions for which storage stability of residues was demonstrated or analysed within 1 month. Significant decline of residues during storage of the trial samples is not expected.
The residue trials were evaluated in accordance with the European guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs (European Commission, 2017).
For tree nuts, cocoa beans and raisins, available residue trials are sufficient to derive tentative MRL and risk assessment values, taking note of the following considerations:
Tree nuts: During the peer review, it was highlighted that the available residue trials in tree nuts were small‐scale trials; thus, additional residue studies to simulate the commercial treatment (i.e. fumigation of large containers or stacks with greater propensity for dead spaces and therefore potentially higher residue levels) were required (EFSA, 2010). In the framework of this review, four additional trials on hazelnuts performed in containers were submitted. The data gap identified previously was sufficiently addressed. Therefore, no additional trials are required.
In the trials on pistachio, pecans and almonds, residues were reported in whole nuts and it is not clear whether the nuts were treated and analysed with or without the shell. Nevertheless, residue data on a wide number of tree nut species as reported by the RMS (Austria, 2019) indicated that residue levels in shelled and unshelled commodities do not differ to a significant extent suggesting that sulfuryl fluoride easily penetrates the outer lignified layer of the nuts. Therefore, the information on whether the treatment was done on shelled or unshelled nuts is not considered relevant and the available data are considered acceptable.
Cocoa beans: a residue study conducted on six sets of cocoa beans and performed in the USA was evaluated by Belgium as zonal RMS. Duplicates of 4 kg cocoa beans placed in chambers of 220 litres (including dead space) were fumigated six sequential times at 750 g h/m3. Sampling was conducted after each fumigation. A 4–6 h active aeration period and one of 20–30 h passive aeration were respected before sampling (Belgium, 2013). Residues of sulfuryl fluoride and fluoride ion measured after the first application were considered to support the authorised use. According to the zonal RMS, since cocoa beans were placed in separate compartments of the fumigation chamber, the study should be considered as consisting of six independent trials. Nevertheless, a clear conclusion on whether the results of the study could be considered independent was not reported in the re‐registration report. Moreover, the description of the study available in the re‐registration report is not detailed enough to verify the independence of the trials.
Based on the above considerations, the available data are considered on a tentative basis only and should be confirmed by a more detailed evaluation report allowing to conclude that results can be considered independent. Alternatively, three additional independent trials are still required.
Raisins: according to the GAP reported during the MRL review, sulfuryl fluoride is authorised for fumigation of dry raisins. In order to support this use, three residue trials performed on raisins and analysing for sulfuryl fluoride and fluoride ion were submitted. EFSA considered the available trials sufficient to derive a tentative MRL for raisins. However, one additional GAP compliant trial analysing both for sulfuryl fluoride and fluoride ion should still be requested to support this use and to have a sufficiently robust database to derive an MRL that reflects the residue levels that are expected under realistic conditions. The required trial should be performed simulating the commercial treatment, i.e. fumigation of large containers or stacks with greater propensity for dead spaces and therefore potentially higher residue levels. A risk management decision needs to be taken, how to implement the tentative MRL in the MRL Regulation, considering that currently MRLs are not established for processed products like dry raisins.
For cereals, based on the data provided in the context of previous assessments (EFSA, 2010, 2015) and in the framework of this MRL review (Austria, 2019), the following conclusions are derived:
The authorised uses are on empty cereal mills, empty storage buildings for cereals or empty storage building for flour and bran. The GAP does not specify in detail the different cereals that are to be processed in the mills or that are stored in the storage building. Furthermore, detailed information on the types of mills (dry mills, wet mills) and the milling products is not reported. Contamination of food should not occur, if appropriate risk mitigation measures are implemented in the storage facilities and mills.
Sulfuryl fluoride: a no residue situation can be reasonably assumed according to the conditions of use and the properties of the active substance. Therefore, the MRLs should be set at the LOQ of 0.01 mg/kg for cereals grain; the same MRL would be appropriate for the milled cereal products, i.e. flour, bran, wheat germ and all other milling products.
Fluoride ion: in support of the existing GAP, residue trials were provided where mills and storage rooms for cereals were treated at 290–1,600 g h/m3; overall, the available trials were underdosed compared to the use under assessment (1,500 g h/m3 per treatment) or were not performed on empty mills (grain and processed products were placed in bags and present in the mills during the fumigation). Due to the nature of the residue trials, scaling of the results is not possible. No information was reported on the cleaning procedures of mills prior to the fumigation. According to these trials, high fluoride residue levels in flour, bran and wheat germ occurred after the production in a treated mill structure had been taken up again (up to 86 mg/kg in wheat flour, up to 90 mg/kg in wheat bran and up to 17 mg/kg in wheat germ) (United Kingdom, 2004, 2015). Studies on the levels of fluoride ion in other milling products (such as polished rice, grits, flakes, starch etc.) following fumigation according to the authorised uses on empty mills and storage room are not available.
In the framework of the confirmatory data assessment, residues trials were submitted showing residues lower than 2 mg/kg in flour, bran and wheat germ samples, 60 min after start‐up of the fumigated mill, with the exception of one bran sample of 2.7 mg/kg at 60 min and after 80 min less than the limit of determination (1 mg/kg) with the exception of one flour sample at 120 min (1.4 mg/kg). On the basis of these studies, the applicant proposed the following risk mitigation measure to ensure fluoride residues in flour, bran and wheat germ to remain below the limit of determination (1 mg/kg): the first 20 min of production (flour, bran and wheat germ) must be passed through all machinery/pipelines, collected and sent to landfill. The next 60 min of production (flour, bran and wheat germ) must be collected and sent for reprocessing. Discarded flour, bran and wheat germ must not be used for human or animal consumption (United Kingdom, 2015).
According to EFSA, the available data are not sufficient to derive an MRL that reflects the residue levels expected under realistic conditions in cereals grains and milling products when sulfuryl fluoride is used according to the authorised GAP on empty mills. The data are also not appropriate to confirm that the risk mitigation measure proposed by the applicant is efficient to avoid contamination of milling products.
Therefore, additional trials on cereals compliant with the most critical GAP on empty mills are still required. These additional trials should be representative for different kinds of milling technologies relevant for cereals; they should be performed with adequate analytical method validated for an LOQ that would allow to estimate the background levels for fluoride naturally occurring in cereals (see also Section 1.2.2). Since contamination of food (e.g. milling products, stored products) may occur if the mill machinery/storage rooms were not completely emptied before the fumigation, the cleaning procedures should be reported in the residue trials to verify that they are representative for the GAP (structural treatment of empty mills, empty storage building and storage rooms).
Considering the data gaps related to the toxicological profile of fluoride (see Section 3) and the lack of a sufficiently robust database to derive an MRL, it seems appropriate to set the MRL tentatively at the natural background levels measured in cereals grain and milling products (see Section 1.2.2) to avoid additional dietary exposure of consumers to fluoride residues. Hence, EFSA proposes to set the following MRLs for fluoride ion:
2 mg/kg in cereals grain and flour, 4 mg/kg in cereals bran, 1 mg/kg in wheat germ and maize starch, 0.5 mg/kg in other cereals milling products (semolina, wheat middlings, maize meal, polished rice, maize oil and maize grits), which are the levels measured in the control samples of the available residue trials as reported by the RMS in the framework of this MRL review (Austria, 2019).
For other cereal milling products, no data are currently available, and it was not possible to propose even a tentative MRL.
Food business operators need to ensure that these limits are not exceeded by defining appropriate measures equivalent to the HACCP principles as laid down in Article 5 of Regulation (EC) No 852/2004 of the European Parliament and of the Council which are in accordance with the provisions of Regulation (EC) No 396/2005.
A risk management decision needs to be taken, how to implement the tentative MRLs in the MRL Regulation, considering that currently MRLs are not established for processed products like cereals milling products.
1.2.2. Magnitude of fluoride naturally occurring in plant commodities
Fluoride ion is naturally occurring in plant commodities; thus, information on the background levels on the commodities under assessment in this reasoned opinion would allow to estimate the additional contribution from the use of sulfuryl fluoride to the overall fluoride exposure. In addition, information on background levels is necessary to set MRLs for products for which no GAP was reported.
For the residue trials conducted in treated mills, data on the concentration of fluoride ion in control samples were provided for cereals prior to fumigation; in addition, data for tree nuts analysed prior fumigation were also available (Austria, 2019). Residues in controls were always below the LOQs of 2.2 mg/kg in tree nuts,2 mg/kg in flour and in cereals grain (wheat, barley, maize, rice), 1 mg/kg in wheat germ and maize starch, 0.5 mg/kg in semolina, wheat middlings, maize meal, polished rice, maize oil, maize grits and 4 mg/kg in bran (Austria, 2019). Results from control samples were not available for raisins and cocoa beans.
Information on the background levels of fluoride was also previously reported in the scientific opinion of the Panel on Dietetic Products, Nutrition and Allergies (NDA) (EFSA, 2013); fluoride content in fresh food is generally low (0.1–0.5 mg/kg) except when food is prepared with fluoridated water. Specifically, in plant commodities, the fluoride content ranges between 0.02 and 0.2 mg/kg in fruits and vegetables;0.1–0.29 mg/kg in bread, cereals and cereal meals. For dried plant products, higher residue concentrations were reported (2 mg/kg in dried herbs and 400 mg/kg in dried tea).
These data suggest that in addition to the commodities assessed in the current application, the following MRLs should be set for untreated products to cover the naturally occurring background levels:
0.2 mg/kg in fresh fruits and vegetables, 2 mg/kg in herbal infusions and 400 mg/kg in tea.
Considering that in the scientific opinion of the NDA panel detailed information on the background levels are not available, these MRLs should be considered tentative only and should be confirmed by a more robust data set. Moreover, for dry pulses, oilseeds, oil fruits, coffee beans, cocoa beans, carobs, hops, spices and sugar plants, no information on the background levels is available. Therefore, it was not possible to derive an MRL reflecting the background level for these commodities.
It is noted that for cereals grain according to the data from the scientific opinion of the NDA Panel, an MRL of 0.3 mg/kg would be sufficient to cover the background levels of fluoride in these commodities, which is lower than the residues in the control samples of the residue trials. Consequently, also for processed cereal products, lower levels than the tentative MRL proposals might be appropriate. Hence, additional data are still required to allow an estimation of the background levels for fluoride naturally occurring in cereals (see also Section 1.2.1).
1.2.3. Magnitude of residues in rotational crops
Investigation of sulfuryl fluoride residues in rotational/succeeding crops is not of relevance for the post‐harvest treatments.
1.2.4. Magnitude of residues in processed commodities
No processing studies are currently available and are not required as they are not expected to affect the outcome of the risk assessment. According to the authorised uses reported during the MRL review, sulfuryl fluoride is used for fumigation of raisins and in empty stores and mills where cereals are processed and stored. Therefore, tentative MRLs were derived for processed commodities as raisins and cereals milling products (see Section 1.2.1).
1.2.5. Proposed MRLs
The data from the available residue trials are considered sufficient to calculate MRL and risk assessment values for the first residue definition (i.e. sulfuryl fluoride) for all commodities under evaluation.
As regards the second residue definition (fluoride ion), EFSA considered the results of the trials and the information on the background levels of fluoride naturally occurring in plant commodities. Based on the available data, it was possible to derive MRL proposals and risk assessment values for all plant commodities, except for dry pulses, oilseeds, oil fruits, coffee beans, carobs, hops, spices and sugar plants for which no information on the background levels nor residue trials were available.
EFSA calculated MRL recommendations for raisins and cereals milling products for further risk management considerations, noting that according to Regulation (EC) No 396/2005, the setting of MRLs is not foreseen for processed products.
Considering the data gaps related to the nature of residues in the treated crops and to sufficiently validated analytical methods for enforcement in cocoa beans, in acidic and high‐water content commodities, in tea and in herbal infusions, the missing residue trials on cereals and raisins, and pending more detailed information on the residue study on cocoa beans and on the background levels, all calculated MRLs should be considered tentative only.
2. Residues in livestock
Sulfuryl fluoride is authorised for treatment of empty cereal mills, empty storage buildings for cereals or empty storage buildings for flour and bran. Contamination of feed items (i.e. cereals and its by‐products) should not occur if appropriate risk mitigation measures are implemented in the storage facilities and mills. Consequently, livestock is not expected to be exposed to residues of sulfuryl fluoride and fluoride ion via feed at levels above the LOQ or above the background levels. A further investigation of residues in livestock is therefore not required.
For fluoride ion, the MRL should be set at a level that covers the naturally occurring background concentrations. According to the scientific opinion of the Panel on Dietetic Products, Nutrition and Allergies (NDA) (EFSA, 2013), products of animal origin may contain fluoride ion between 0.05 and 0.15 mg/kg in milk and dairy, 0.15–0.29 mg/kg in meat and meat products and 0.18 mg/kg in eggs. Based on these data, the following MRL proposals are derived: 0.2 mg/kg in milks and eggs and 0.3 mg/kg in livestock tissues. These MRLs should be considered tentative only and should be confirmed by more detailed information on the background levels. Moreover, an analytical method for the enforcement of fluoride ion in animal commodities is not available.
3. Consumer risk assessment
In the framework of this review, the existing uses of sulfuryl fluoride reported by the RMS in Appendix A were considered.
It is highlighted that only an indicative consumer risk assessment for sulfuryl fluoride and an indicative exposure assessment for fluoride ion could be performed, pending a final decision on the residue definitions for risk assessment (see Section 1.1.6). These indicative assessments were based on the tentative residue definitions (i.e. parent sulfuryl fluoride and fluoride ion, expressed individually).
The use of sulfuryl fluoride was previously assessed by the JMPR (FAO, 2005). Following this assessment by JMPR, the CAC adopted CXLs for the residue definition sulfuryl fluoride. Codex MRLs are not set for fluoride ion; JMPR considered fluoride ion as relevant for dietary intake and therefore some information on the occurrence of fluoride ion in treated crops is available in the JMPR evaluation. The CXLs for sulfuryl fluoride are international recommendations that need to be considered by European risk managers when establishing MRLs. To allow taking an informed risk management decision on the possible implementation of CXLs in the EU legislation, EFSA calculated risk assessment scenarios with and without consideration of the existing CXLs.
The toxicological profile of sulfuryl fluoride was assessed in the framework of the peer review under Directive 91/414/EEC and the data were sufficient to derive an acute reference dose (ARfD) of 0.7 mg/kg body weight (bw) and an acceptable daily intake (ADI) value of 0.014 mg/kg bw per day (European Commission, 2016).
The assumptions and the results for the risk assessment for sulfuryl fluoride are reported in Sections 3.1.1 (scenario without CXLs) and 3.2.1 (scenario with CXLs).
In the framework of the peer review, the toxicological data for fluoride ion were found to be insufficient to derive toxicological reference values (EFSA, 2010). Lacking EU values, EFSA explored whether other information on the toxicological properties of fluoride was available that could be used in assessing consumer health risks related to fluoride exposure resulting from the uses of sulfuryl fluoride:
In 2005, the EFSA Scientific Panel on Dietetic Products, Nutrition and Allergies derived an upper tolerable intake level (UL) of 0.1 mg/kg bw for fluoride for children up to 8 years which is equivalent to 1.5 and 2.5 mg fluoride per day in children aged 1–3 years and 4–8 years, respectively, and to 5 and 7 mg fluoride per day in children aged 9–14 years and adults (EFSA, 2005).
In 2013, the EFSA Panel on Dietetic Products, Nutrition and Allergies (EFSA, 2013) derived the adequate intake (AI) of 0.05 mg/kg bw per day. This dietary reference value (DRV) is the average nutrient level consumed daily by a typical healthy population that is assumed to be adequate for the population's needs.
According to the WHO (WHO, 2006), the minimum oral dose that may produce signs of acute fluoride intoxication is 1 mg of fluoride per kg of body weight.
EFSA performed an indicative calculation of the short‐term exposure for the commodities under assessment and to compare the result with the oral dose defined by the WHO that may cause intoxication (margin of exposure).
For assessing risks related to long‐term effects, EFSA compared the expected exposure with the DRV derived by EFSA in 2013 (estimated exposure expressed as % of the DRV); in addition, EFSA calculated the margin of exposure for children and adults to the upper tolerable intake level derived by EFSA in 2005.
The assumptions and the results for these calculations are reported in Sections 3.1.2 (without consideration of uses assessed by JMPR) and 3.2.2 (with consideration of uses assessed by JMPR).
3.1. Consumer risk assessment without consideration of the existing CXLs
3.1.1. Sulfuryl fluoride
Chronic and acute exposure calculations for all crops reported in the framework of this review were performed using revision 3.1 of the EFSA PRIMo (EFSA, 2019, 2018). Input values for the exposure calculations were derived in compliance with the decision tree reported in Appendix E. Hence, for those commodities where a tentative MRL for sulfuryl fluoride was derived by EFSA in the framework of this review, input values were derived according to the internationally agreed methodologies (FAO, 2009).
The acute exposure calculations were only performed for the products under assessment (tree nuts, raisins, cereal grain and milling products and cocoa beans).
Lacking detailed consumption data for processed products to be used for chronic exposure assessments, the chronic exposure for cereal products and raisins was calculated based on the supervised trials median residue (STMR) values derived from cereal flour and raisins which were combined with the consumption data for unprocessed cereal and fresh table grapes, respectively. STMR from cereal flour was used as input value for the chronic assessment of all processed commodities based on cereals, considering that most of the consumed cereal products are derived from a milled product which is then further processed. For raisins, the STMR derived for the dried fruit was recalculated to grapes, assuming a dilution factor of 4.7 to accommodate for the lower dry matter content in fresh grapes. The exposure values calculated according to these assumptions are conservative and are expected to lead to an overestimation.
All input values included in the exposure calculations are summarised in Appendix D.1.
The calculated exposure was compared with the toxicological reference values for sulfuryl fluoride, derived by EFSA (EFSA, 2010). The highest acute exposure was calculated for pistachios, representing 4% of the ARfD. The highest chronic exposure was calculated for IE adult, representing 4% of the acceptable daily intake (ADI).
3.1.2. Fluoride ion
The chronic and acute exposure calculations for fluoride were also based on the algorithm and the consumption data implemented in PRIMo revision 3.1. (EFSA, 2019, 2018). However, due to the specific situation, adaptations of the calculations were required as outlined below.
All input values included in these calculations are summarised in Appendix D.1.
The short‐term exposure was calculated only for the commodities under assessment, assuming that they are not consumed together. The highest residue levels from the available residue trials in tree nuts (except coconuts), cocoa beans, raisins, cereals and cereals milling products were used as input values.
According to this calculation, the highest acute exposure among the food products for which the calculation was performed was identified for pistachios consumed by children accounting for 0.122 mg/kg bw; the margin of exposure to the minimum oral dose which was found to produce signs of acute fluoride intoxications (i.e. 1 mg/kg bw) (WHO, 2006) was calculated to be 8.2 (see Table 1).
Table 1.
Results of the calculation of the margin of short‐term exposure for the commodities under assessment
| Commodity | Exposure EU uses (children) (μg/kg bw) | Minimum oral dose producing signs of acute fluoride intoxications (μg/kg bw) | MoE EU uses |
|---|---|---|---|
| Pistachios | 122 | 1,000 | 8.2 |
| Chestnuts | 88 | 1,000 | 11.4 |
| Walnuts | 71 | 1,000 | 14.1 |
| Hazelnuts/cobnuts | 69 | 1,000 | 14.6 |
| Almonds | 61 | 1,000 | 16.5 |
| Pecans | 58 | 1,000 | 17.3 |
| Cashew nuts | 53 | 1,000 | 18.8 |
| Wheat | 29 | 1,000 | 34.6 |
| Rice | 25 | 1,000 | 39.7 |
| Brazil nuts | 18 | 1,000 | 54.8 |
| Maize/corn | 13 | 1,000 | 74.2 |
| Rye | 13 | 1,000 | 79.1 |
| Cocoa beans | 12 | 1,000 | 82.4 |
| Macadamia | 12 | 1,000 | 86.8 |
| Barley | 11 | 1,000 | 89.1 |
| Buckwheat and other pseudo‐cereals | 10 | 1,000 | 100 |
| Pine nut kernels | 6.9 | 1,000 | 144 |
| Sorghum | 6.4 | 1,000 | 156 |
| Common millet/proso millet | 2.8 | 1,000 | 361 |
| Oat | 2.2 | 1,000 | 451 |
| Wheat/milling (flour) | 24 | 1,000 | 41.4 |
| Wheat/milling (wholemeal)‐baking | 11 | 1,000 | 90.2 |
| Millet/boiled | 11 | 1,000 | 92.2 |
| Buckwheat/bulgur and grits | 11 | 1,000 | 93.2 |
| Rice/milling (polishing) | 7.6 | 1,000 | 131 |
| Rye/boiled | 7.3 | 1,000 | 138 |
| Oat/boiled | 7.3 | 1,000 | 138 |
| Buckwheat/boiled | 7.3 | 1,000 | 138 |
| Barley/cooked | 7.3 | 1,000 | 138 |
| Rye/milling (wholemeal)‐baking | 7.0 | 1,000 | 142 |
| Oat/milling (flakes) | 6.0 | 1,000 | 167 |
| Cocoa (fermented beans)/processed (not specified) | 4.5 | 1,000 | 221 |
| Maize/processed (not specified) | 4.3 | 1,000 | 235 |
| Barley/milling (flour) | 3.6 | 1,000 | 276 |
| Maize/oil | 0.5 | 1,000 | 2147 |
bw: body weight; MoE: margin of exposure.
To assess whether the additional contribution of fluoride ion resulting from the use of sulfuryl fluoride to the overall fluoride exposure is likely to pose a long‐term risk to consumers, EFSA calculated different scenarios:
Scenario 1: background exposure based only on the background levels of fluoride;
Scenario 2: background exposure plus exposure resulting from fumigation with sulfuryl fluoride according to the GAPs reported in Appendix A.
The following input values were used in PRIMo revision 3.1 for the two scenarios:
-
Scenario 1 – background exposure based only on the background levels of fluoride
-
–
Fluoride background levels for fruits, vegetables, dried herbs, tea and animal commodities reported in the scientific opinion of the Panel on Dietetic Products, Nutrition and Allergies (NDA) (EFSA, 2013);
-
–
Fluoride concentrations measured in untreated control samples of the residue trials on tree nuts, cereals (Austria, 2019);
-
–
Existing EU MRLs (Reg. (EC) No 839/2008) for all other commodities (except cocoa beans),10 assuming that they reflect the background levels.
-
–
-
Scenario 2 – background exposure plus exposure resulting from fumigation with sulfuryl fluoride according to the GAPs reported in Appendix A
-
–
The median residue levels of the residue trials in tree nuts (except coconuts) and cocoa beans;
-
–
For coconuts, fluoride concentrations measured in untreated control samples of the residue trials on tree nuts (Austria, 2019);
-
–
For processed commodities (raisins and milled cereal products), the same approach as for the chronic risk assessment for sulfuryl fluoride was applied;
-
–
Background levels reported in scientific opinion of the Panel on Dietetic Products, Nutrition and Allergies (NDA) (EFSA, 2013), for fruits, vegetables, dried herbs, tea and animal commodities;
-
–
Existing EU MRLs (Reg. (EC) No 839/2008) were considered for all other commodities as done in scenario 1.
-
–
In scenario 1, the highest chronic exposure was calculated for French adults, accounting for 0.069 mg/kg bw per day while in scenario 2, the highest chronic exposure was calculated for Irish adults at 0.072 mg/kg bw per day indicating that the contribution of the fluoride from the authorised uses to the background exposure is low. The estimated exposure in scenario 1 and scenario 2 accounted for 138% and 145% of the DRV (AI, i.e. 0.05 mg/kg bw per day) derived by EFSA NDA Panel (EFSA, 2013) (see Appendix C). These calculations give an indication that in both scenarios, the fluoride exposure exceeds the average nutrient level adequate for the population's needs.
It is noted that the results of the exposure assessment in both scenarios are likely to overestimate the real exposure, considering that for a wide range of commodities, the calculations were performed with the MRL, which is set at the upper level of the background levels, while consumers are likely to be exposed to residue concentrations that are characterised by a distribution, with a median value which is lower than the MRL. The results provide an indication that the contribution of commodities treated with sulfuryl fluoride according to the uses assessed in this evaluation is low, compared to the calculated background exposure.
The margin of exposure to the upper tolerable intake was calculated for the French diet (adults), Irish adults, Dutch toddler and Dutch children being the European diets with the highest estimated fluoride exposure. According to the results of these calculations, the margin of exposure in scenario 2 was slightly lower than for scenario 1, supporting the conclusion that the contribution of commodities treated with sulfuryl fluoride according to the uses assessed in this evaluation is low, compared to the calculated background exposure (see Table 2).
Table 2.
Results of the calculation of the margin of long‐term exposure according to scenario 1 and 2, respectively
| Diet | Bw (kg) | Exposure scenario 1 (μg/kg bw per day) | Exposure scenario 2 (μg/kg bw per day) | UL (μg/kg bw per day) | MoE scenario 1 | MoE scenario 2 |
|---|---|---|---|---|---|---|
| French adult | 66.4 | 69 | 69 | 100 | 1.45 | 1.44 |
| Irish adult | 75.2 | 68 | 72 | 100 | 1.47 | 1.38 |
| Dutch toddler | 10.2 | 65 | 65 | 100 | 1.55 | 1.54 |
| Dutch child | 18.4 | 48 | 49 | 100 | 2.09 | 2.02 |
bw: body weight as reported in the PRIMo file; UL: upper tolerable intake level; MoE: margin of exposure.
3.2. Consumer risk assessment with consideration of the existing CXLs
EFSA also considered the existing CXLs for sulfuryl fluoride, to verify whether they could be implemented in the EU legislation. Although no specific CXLs are set for the fluoride ion, both sulfuryl fluoride and fluoride ion were analysed in the trials considered by the JMPR to derive the CXLs. In order to support risk managers, EFSA considered data from the JMPR to calculate the corresponding MRL proposals for fluoride ion for commodities for which CXLs are established. The MRLs were calculated rounding up the highest residue of fluoride found in the trials supporting the uses of sulfuryl fluoride assessed by the JMPR, to the next MRL class.
It is noted that CXLs for cereals grain and milling products are based on direct fumigation of these commodities, without implementing any risk mitigation measures to reduce the occurrence of fluoride residues (no discard of the first milling fraction or re‐processing). According to these uses, fluoride ion is present at significant levels in the treated products (up to 104 mg/kg in wheat germ) and significant consumer and livestock exposure cannot be excluded (FAO, 2005).
3.2.1. Sulfuryl fluoride
For the risk assessment scenarios considering the existing CXLs for sulfuryl fluoride, EFSA replaced the input values described in Section 3.1.1 for coconuts, cereals and raisins by the median residue/highest residue derived by JMPR.
The input values used for this exposure calculation are also provided in Appendix D.2.
Acute and chronic exposure calculations were also performed using revision 3.1 of the EFSA PRIMo and the exposure values calculated were compared with the toxicological reference values derived for sulfuryl fluoride. The highest acute exposure was calculated for coconuts, representing 5% of the ARfD. The highest chronic exposure was calculated for IE adult diet, representing 4% of the ADI.
3.2.2. Fluoride ion
For the acute exposure assessment, EFSA included in the calculation the HR derived by JMPR for coconuts, cereals and milling products and raisins.
In the chronic exposure assessment (scenario 3), EFSA updated the scenario 2, replacing the input value for raisins, coconuts and cereals with the STMR derived by JMPR. As done in the previous scenarios, STMR from cereal flour was used as input value for the chronic assessment of all processed commodities based on cereals, considering that most of the consumed cereal products are derived from a milled product which is then further processed.
An overview of the input values used for this exposure calculation is also provided in Appendix D.2.
The results of these calculations are reported in Tables 3 and 4, respectively.
Table 3.
Results of the calculation of the margin of short‐term exposure for the commodities under assessment and the CXLs
| Commodity | Exposure EU uses and CXLs (children) (μg/kg bw) | Minimum oral dose producing signs of acute fluoride intoxications (μg/kg bw) | MoE EU uses and CXLs |
|---|---|---|---|
| Wheat | 361 | 1,000 | 2.8 |
| Rice | 315 | 1,000 | 3.2 |
| Maize/corn | 168 | 1,000 | 5.9 |
| Rye | 158 | 1,000 | 6.3 |
| Barley | 140 | 1,000 | 7.1 |
| Coconuts | 131 | 1,000 | 7.6 |
| Buckwheat and other pseudo‐cereals | 124 | 1,000 | 8.0 |
| Pistachios | 122 | 1,000 | 8.2 |
| Chestnuts | 88 | 1,000 | 11.4 |
| Sorghum | 80 | 1,000 | 12.5 |
| Walnuts | 71 | 1,000 | 14.1 |
| Hazelnuts/cobnuts | 69 | 1,000 | 14.6 |
| Almonds | 61 | 1,000 | 16.5 |
| Pecans | 58 | 1,000 | 17.3 |
| Cashew nuts | 53 | 1,000 | 18.8 |
| Common millet/proso millet | 35 | 1,000 | 28.9 |
| Oat | 28 | 1,000 | 36.0 |
| Brazil nuts | 18 | 1,000 | 54.8 |
| Cocoa beans | 12 | 1,000 | 82.4 |
| Macadamia | 12 | 1,000 | 86.8 |
| Pine nut kernels | 6.9 | 1,000 | 144 |
| Wheat/milling (flour) | 665 | 1,000 | 1.5 |
| Wheat/milling (wholemeal)‐baking | 305 | 1,000 | 3.3 |
| Rye/milling (wholemeal)‐baking | 193 | 1,000 | 5.2 |
| Maize/processed (not specified) | 149 | 1,000 | 6.7 |
| Coconuts/drink | 129 | 1,000 | 7.8 |
| Rice/milling (polishing) | 128 | 1,000 | 7.8 |
| Millet/boiled | 114 | 1,000 | 8.8 |
| Buckwheat/bulgur and grits | 113 | 1,000 | 8.9 |
| Barley/milling (flour) | 100 | 1,000 | 10.0 |
| Rye/boiled | 76 | 1,000 | 13.1 |
| Oat/boiled | 76 | 1,000 | 13.1 |
| Buckwheat/boiled | 76 | 1,000 | 13.1 |
| Barley/cooked | 76 | 1,000 | 13.1 |
| Oat/milling (flakes) | 63 | 1,000 | 15.9 |
| Maize/oil | 20 | 1,000 | 51.1 |
| Cocoa (fermented beans)/processed (not specified) | 4.5 | 1,000 | 221 |
bw: body weight; MoE: margin of exposure.
Table 4.
Results of the calculation of the margin of long‐term exposure according to scenario 3 (including all CXLs) and 4 (excluding the CXLs for cereals), respectively
| Diet | Bw (kg) | Exposure scenario 3 (μg/kg bw per day) | Exposure scenario 4 (μg/kg bw per day) | UL (μg/kg bw per day) | MoE scenario 3 | MoE scenario 4 |
|---|---|---|---|---|---|---|
| French adult | 66.4 | 151 | 70 | 100 | 0.66 | 1.44 |
| Irish adult | 75.2 | 183 | 72 | 100 | 0.55 | 1.38 |
| Dutch toddler | 10.2 | 481 | 66 | 100 | 0.21 | 1.52 |
| Dutch child | 18.4 | 208 | 50 | 100 | 0.48 | 2.01 |
bw: body weight as reported in the PRIMo file; UL: upper tolerable intake level; MoE: margin of exposure.
The highest short‐term exposure among the food products for which the calculation was performed was identified for wheat flour consumed by children accounting for 0.665 mg/kg bw; the margin of exposure to the minimum oral dose which was found to produce signs of acute fluoride intoxications (i.e. 1 mg/kg bw) (WHO, 2006) was calculated to be 1.5 (see Table 3).
In scenario 3, the highest long‐term exposure was calculated for Dutch toddler, accounting for 0.481 mg/kg bw per day, being higher compared to the highest exposures calculated according to scenarios 1 and 2 (0.069 and 0.072 mg/kg bw per day, respectively). The estimated exposure in scenario 3, including all the CXLs, accounted for 962% of the DRV (AI, i.e. 0.05 mg/kg bw per day) derived by EFSA NDA Panel (EFSA, 2013), being higher compared to the exposure calculated considering the EU uses only (accounting for 145% of the DRV) (see Appendix C). The margin of exposure in scenario 3 was below 1 (see Table 4). These calculations give an indication that the contribution of the fluoride ion from the uses assessed by the JMPR cannot be considered negligible.
EFSA therefore performed a new calculation (scenario 4), disregarding the CXLs for cereals. The highest exposure calculated according to this scenario is slightly higher compared to scenario 1 and scenario 2 (see Tables 4 and 2). This calculation indicates that the contribution of fluoride ion resulting from the uses of sulfuryl fluoride on raisins and coconuts assessed by the JMPR is low compared to the background exposure.
3.3. Overall conclusion of the consumer risk assessment
According to the residue trials assessed in the framework of this MRL review, significant residues of fluoride ion are expected in the commodities under assessment when sulfuryl fluoride is used according to the most critical GAPs currently authorised.
Although according to this indicative calculation the contribution from the authorised uses and CXLs to the overall fluoride exposure is low (except for the uses on cereals assessed by the JMPR), a final risk assessment could not be performed due to the following main data gaps:
lack of agreed toxicological reference values for fluoride ion;
lack of comprehensive data on background levels for fluoride ion in plant and animal products.
Therefore, an ‘overall’ conclusive consumer exposure assessment for the uses under consideration could not be performed.
Conclusions
The nature of sulfuryl fluoride in plant commodities was not investigated in metabolism studies. Instead, information from public literature on the mode of degradation of sulfuryl fluoride in several food commodities was available. However, this information was considered not sufficient to fully elucidate the nature of the residues expected in food matrices upon fumigation. Tentative residue definitions for enforcement and risk assessment were proposed, i.e. sulfuryl fluoride and fluoride ion expressed individually. Depending on the results of the additional studies elucidating the nature of residues in unprocessed products, further information might be required also for processed products (investigation of hydrolytic stability of metabolites identified in treated products); hence, the residue definitions for processed products are also set on a tentative basis. Since sulfuryl fluoride is authorised only as fumigation on the interior of buildings, studies on rotational crops are not relevant.
Fully validated analytical methods are available for the enforcement of the proposed residue definitions in high oil and dry matrices but also in dried fruits at the LOQs of 0.004–0.01 mg/kg for sulfuryl fluoride and 0.05–5 mg/kg for fluoride ion. Analytical methods for the enforcement of fluoride ion in acidic and high‐water content commodities, in tea, coffee beans, herbal infusions, carobs, hops and spices are not available. According to the EURLs, an LOQ of 0.005 mg/kg for sulfuryl fluoride is achievable in high water, high oil, dry matrices and tea by using a single residue method. An analytical method for the enforcement of fluoride ion is currently not available to the EURLs. An analytical method for the enforcement of the proposed residue definitions in cocoa beans is not available and is still required.
The data from the available residue trials are considered sufficient to calculate MRL and risk assessment values for the first residue definition (i.e. sulfuryl fluoride) for all commodities under evaluation.
As regards the second residue definition (fluoride ion), EFSA considered the results of the trials and the information on the background levels of fluoride naturally occurring in plant commodities. Based on the available data, it was possible to calculate MRL and risk assessment values for all plant commodities, except for dry pulses, oilseeds, oil fruits, coffee beans, carobs, hops, spices and sugar plants for which no information on the background levels nor residue trials were available.
EFSA calculated MRL recommendations for raisins and cereals milling products for further risk management considerations, noting that according to Regulation (EC) No 396/2005, the setting of MRLs is not foreseen for processed products.
Considering the data gaps related to the nature of residues in the treated crops and to sufficiently validated analytical methods for enforcement in cocoa beans, in acidic and high‐water content commodities, in tea and in herbal infusions, the missing residue trials on cereals and raisins, and pending more detailed information on the residue study on cocoa beans and on the background levels, all calculated MRLs should be considered tentative only.
Sulfuryl fluoride is authorised for use on cereals that might be fed to livestock. Nevertheless, considering that the authorised use is on empty stores and mills and the risk mitigation currently in place, livestock are not expected to be exposed to residues of sulfuryl fluoride and fluoride ion above the LOQ or above the background levels. Therefore, there is no need to further investigate residues in livestock. Regarding background concentrations from other sources than the authorised uses, data from the scientific opinion of the Panel on Dietetic Products, Nutrition and Allergies on fluoride were considered to derive MRLs for animal commodities. Nevertheless, these MRLs should be considered tentative only and should be confirmed by more detailed information on the background levels. Moreover, an analytical method for the enforcement of fluoride ion in animal commodities is not available.
Chronic and acute exposure calculations for all crops reported in the framework of this review were performed using revision 3.1 of the EFSA PRIMo for both sulfuryl fluoride and fluoride ion.
The exposure values calculated were compared with the toxicological reference values for sulfuryl fluoride, derived by EFSA. The highest chronic exposure was calculated for IE adult, representing 4% of the ADI and the highest acute exposure was calculated for pistachios, representing 4% of the ARfD. Apart for the MRLs evaluated in the framework of this review, internationally recommended CXLs have also been established for sulfuryl fluoride. Additional calculations of the consumer exposure, considering these CXLs, were therefore carried out. The highest chronic exposure represented 4% of the ADI (IE adults) and the highest acute exposure amounted to 5% of the ARfD (coconuts).
For fluoride ion, a standard consumer risk assessment could not be performed, lacking information on the toxicological reference values. Nevertheless, considering that fluoride ion is naturally occurring in food of plant and animal origin, EFSA performed an indicative calculation of the consumer exposure to estimate whether the uses currently authorised will contribute significantly to the overall consumer exposure to fluoride ion. According to these indicative calculations, the contribution from the authorised uses and CXLs to the overall fluoride exposure is low (except for the uses on cereals assessed by the JMPR). Nevertheless, an ‘overall’ conclusive consumer exposure assessment for the uses under consideration could not be performed, pending additional information on the toxicological profile of fluoride ion and comprehensive data on background levels for fluoride ion in plant and animal products.
Recommendations
MRL recommendations for sulfuryl fluoride and fluoride ion were derived in compliance with the assessment described above. None of the MRL values listed in the table are recommended for inclusion in Annex II to the Regulation as they are not sufficiently supported by data (see Table 5). In particular, all the proposed MRLs need to be confirmed by the following data:
Studies to investigate/elucidate the nature of the residues in food matrices upon fumigation with sulfuryl fluoride and to verify whether additional compounds are formed in food matrices following fumigation with sulfuryl fluoride. Depending on the outcome of these studies, further toxicological data and further studies investigating the nature of residue in processed commodities, may be required.
Additional studies addressing the general toxicity profile of fluoride ion.
A detailed evaluation of the study supporting the use on cocoa beans allowing to confirm that the available results are from independent trials. Alternatively, three additional independent trials on cocoa beans are still required.
A fully validated analytical method for the enforcement of sulfuryl fluoride and fluoride ion in cocoa beans.
Detailed information on the natural background levels of fluoride ion in commodities of plant and animal origin.
Additional trials on cereals compliant with the most critical GAP on empty mills taking into consideration different kinds of milling technologies relevant for cereals. These trials should be performed with adequate analytical method validated for an LOQ that would allow to estimate the background levels for fluoride naturally occurring in cereals.
Fully validated analytical methods for the enforcement of fluoride ion in high water content and acidic commodities, tea, coffee beans, herbal infusions, carobs, hops, spices and animal commodities. It is noted that no uses are currently authorised on these commodities. Therefore, risk managers should discuss whether this data gap should be reflected in the EU legislation.
Table 5.
Summary table
| Code number | Commodity | Existing EU MRL (mg/kg) | Existing CXLa (mg/kg) | Outcome of the review | |
|---|---|---|---|---|---|
| MRL (mg/kg) | Comment | ||||
| Enforcement residue definition 1: sulfuryl fluoride | |||||
| 120010 | Almonds | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| 120020 | Brazil nuts | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| 120030 | Cashew nuts | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| 120040 | Chestnuts | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| 120050 | Coconuts | 10 | 3 | 3 |
Further consideration neededc Data gaps #1, 2 |
| 120060 | Hazelnuts | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| 120070 | Macadamia | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| 120080 | Pecans | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| 120090 | Pine nuts | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| 120100 | Pistachios | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| 120110 | Walnuts | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| – | Raisins | – | 0.06 | 0.06 |
Further consideration neededd Data gaps #1, 2 |
| 500000 | Cereals grain | 0.05 | 0.05 | 0.01* |
Further consideration needee Data gaps #1, 2 |
| – | Cereals, milling products | – | 0.1 | 0.01* |
Further consideration needede Data gaps #1, 2 |
| 640000 | Cocoa (fermented beans) | 0.02* | – | 0.03 |
Further consideration neededf Data gaps #1, 2, 3, 4 |
| – | Other commodities of plant and/or animal origin | See Reg. 839/2008 | – | – | Further consideration neededg |
| Enforcement residue definition 2: fluoride ion | |||||
| 110000 | Citrus fruits | – | 0.2 |
Further consideration neededh Data gap #5, 7 |
|
| 120010 | Almonds | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| 120020 | Brazil nuts | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| 120030 | Cashew nuts | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| 120040 | Chestnuts | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| 120050 | Coconuts | 25 | 15 | 15 |
Further consideration neededc Data gaps #1, 2 |
| 120060 | Hazelnuts | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| 120070 | Macadamia | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| 120080 | Pecans | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| 120090 | Pine nuts | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| 120100 | Pistachios | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| 120110 | Walnuts | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| – | Raisins | – | 3 | 3 |
Further consideration neededd Data gaps #1, 2 |
| 130000 | Pome fruits | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 140000 | Stone fruits | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 150000 | Berries and small fruits | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 161010 | Dates | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 161020 | Figs | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 161030 | Table olives | 2* | 0.2 |
Further consideration neededh Data gap #5 |
|
| 161040 | Kumquats | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 161050 | Carambolas | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 161060 | Kaki/Japanese persimmons | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 161070 | Jambuls/jambolans | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 161040 | Kumquats | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 162000 | Miscellaneous fruits (inedible peel, small) | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163010 | Avocados | 2* | 0.2 |
Further consideration neededh Data gap #5 |
|
| 163020 | Bananas | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163030 | Mangoes | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163040 | Papayas | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163050 | Granate apples/pomegranates | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163060 | Cherimoyas | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163070 | Guavas | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163080 | Pineapples | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163090 | Breadfruits | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163100 | Durians | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163110 | Soursops/guanabanas | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 200000 | Vegetables, fresh or frozen | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 300000 | Pulses | 2* | – | 2 |
Further consideration neededi Data gap #5 |
| 400000 | Oilseeds and oil fruits | 2* | – | 2 |
Further consideration neededi Data gap #5 |
| 500000 | Cereals grain | 2* | 25 | 2 |
Further consideration needede Data gaps #1, 2, 5, 6 |
| – | Cereals flour | – | 80 | 2 |
Further consideration needede Data gaps #1, 2, 5, 6 |
| – | Cereals bran | – | 150 | 4 |
Further consideration needede Data gaps #1, 2, 5, 6 |
| – | Wheat germ | – | 150 | 1 |
Further consideration needede Data gaps #1, 2, 5, 6 |
| – | Maize Starch | – | – | 1 |
Further consideration neededf Data gaps #1, 2, 5, 6 |
| – |
Semolina Wheat middlings Maize grits Maize oil |
– | – | 0.5 |
Further consideration neededf Data gaps #1, 2, 5, 6 |
| – |
Maize meal Polished rice |
– | 150 | 0.5 |
Further consideration needede Data gaps #1, 2, 5, 6 |
| 610000 | Tea (dried leaves of Camellia sinensis) | 350 | – | 400 |
Further consideration neededh Data gap #5, 7 |
| 620000 | Coffee beans | 5 | – | 5 |
Further consideration neededi Data gap #5, 7 |
| 630000 | Herbal infusions | 10 | – | 2 |
Further consideration neededh Data gap #5, 7 |
| 640000 | Cocoa (fermented beans) | 10 | – | 5 |
Further consideration neededf Data gaps #1, 2, 3, 4 |
| 650000 | Carobs | 10 | – | 10 |
Further consideration neededi Data gap #5, 7 |
| 700000 | Hops | 10 | – | 10 |
Further consideration neededi Data gap #5, 7 |
| 800000 | Spices | 5 | – | 5 |
Further consideration neededi Data gap #5, 7 |
| 900000 | Sugar plants | 2 | – | 2 |
Further consideration neededi Data gap #5, 7 |
| 1010000 | Products of animal origin, tissues | 1 | – | 0.3 |
Further consideration neededh Data gaps #5, 7 |
| 1020000 | Milks | 0.2 | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 1030000 | Eggs | 0.2 | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
MRL: maximum residue level; CXL: codex maximum residue limit.
Indicates that the MRL is set at the limit of quantification.
The CAC adopted CXLs for the residue definition sulfuryl fluoride only. Although no specific CXLs are set for the fluoride ion, both sulfuryl fluoride and fluoride ion were analysed in the trials considered by the JMPR to derive the CXLs. In order to support risk managers, EFSA considered the data from the JMPR to calculate the corresponding MRL proposals for fluoride ion for commodities for which CXLs are established.
The proposed MRL is based on a GAP evaluated at EU level which is not fully supported by data. Although no risk for consumers has been identified for sulfuryl fluoride, lacking information on the toxicological reference values for fluoride ion, an overall consumer risk assessment could not be performed. The calculation of the margin of exposure indicated that the contribution from the existing EU use on this commodity to the overall fluoride exposure is low. The existing CXL is covered by the proposed MRL.
The proposed MRL is based on the existing CXL which is not fully supported by data. Although no risk for consumers has been identified for sulfuryl fluoride, lacking information on the toxicological reference values for fluoride ion, an overall consumer risk assessment could not be performed. The calculation of the margin of exposure indicated that the contribution from the existing CXL on this commodity to the overall fluoride exposure is low. There are no relevant authorisations or import tolerances reported at EU level.
The proposed MRL is based on the existing CXL which is not fully supported by data. Although no risk for consumers has been identified for sulfuryl fluoride, lacking information on the toxicological reference values for fluoride ion, an overall consumer risk assessment could not be performed. The calculation of the margin of exposure indicated that the contribution from the existing CXL on this commodity to the overall fluoride exposure is low. The GAP authorised at EU level lead to a lower MRL.
The proposed MRL is based on a GAP evaluated at EU level which is not fully supported by data. Although no risk for consumers has been identified for sulfuryl fluoride, lacking information on the toxicological reference values for fluoride ion, an overall consumer risk assessment could not be performed. The calculation of the margin of exposure indicated that the contribution from the existing EU use on this commodity to the overall fluoride exposure is low. The existing CXL is higher, but its contribution to the overall fluoride exposure is higher.
The proposed MRL is based on a GAP evaluated at EU level which is not fully supported by data. Although no risk for consumers has been identified for sulfuryl fluoride, lacking information on the toxicological reference values for fluoride ion, an overall consumer risk assessment could not be performed. The calculation of the margin of exposure indicated that the contribution from the existing EU use on this commodity to the overall fluoride exposure is low. No CXL is available.
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered.
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. As fluoride ion is naturally occurring, a tentative MRL is proposed at background levels.
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. Lacking information on the background levels, the existing MRL is indicatively proposed.
It is highlighted that some of the MRLs derived result from a CXL whereas a GAP reported by the RMS was not fully supported by data. EFSA therefore identified the following data gap which is not expected to impact on the validity of the MRLs derived but which might have an impact on national authorisations:
One additional residue trial on raisins, simulating the commercial treatment i.e. fumigation of large bags/containers/stacks with greater propensity for dead spaces and therefore potential higher residue levels of sulfuryl fluoride and fluoride ion.
If the above reported data gaps are not addressed in the future, Member States are recommended to withdraw or modify the relevant authorisations at national level.
According to the information provided by the EURLs, the analytical standard for sulfuryl fluoride and fluoride ion is commercially available.
It is also underlined that, lacking a sufficiently robust database to derive an MRL that reflects the residue levels expected under realistic conditions in cereals grains and milling products and considering the data gaps related to the toxicological profile of fluoride, proper risk mitigation measure should be implemented in order to avoid residues of fluoride ion in cereals grain and in cereal milling products above the background levels.
Food business operators need to ensure that the proposed MRLs for cereals are not exceeded by defining appropriate measures equivalent to the HACCP principles as laid down in Article 5 of Regulation (EC) No 852/2004 of the European Parliament and of the Council which are in accordance with the provisions of Regulation (EC) No 396/2005.
A risk management decision needs to be taken, how to implement the tentative MRL for processed commodities in the MRL Regulation, considering that currently MRLs are not established for processed products like raisins and dry milling products.
Abbreviations
- a.i.
active ingredient
- AI
adequate intake
- a.s.
active substance
- ADI
acceptable daily intake
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- CAC
Codex Alimentarius Commission
- CAS
Chemical Abstract Service
- CF
conversion factor for enforcement residue definition to risk assessment residue definition
- CIRCA
(EU) Communication & Information Resource Centre Administrator
- CS
capsule suspension
- CTP
Product (P) of concentration (C) and exposure time (T)
- CV
coefficient of variation (relative standard deviation)
- CXL
codex maximum residue limit
- DAR
draft assessment report
- DAT
days after treatment
- DB
dietary burden
- DM
dry matter
- DRV
dietary reference value
- DS
powder for dry seed treatment
- EC
emulsifiable concentrate
- ECD
electron capture detector
- EDI
estimated daily intake
- EMS
evaluating Member State
- EURLs
European Union Reference Laboratories for Pesticide Residues (former CRLs)
- FAO
Food and Agriculture Organization of the United Nations
- GAP
Good Agricultural Practice
- GC
gas chromatography
- GC‐ECD
gas chromatography with electron capture detector
- GC‐FID
gas chromatography with flame ionisation detector
- GC‐MS
gas chromatography with mass spectrometry
- GC‐MS/MS
gas chromatography with tandem mass spectrometry
- CTP
Concentration Time Product
- GS
growth stage
- HACCP
Hazard Analysis and Critical Control Points
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
independent laboratory validation
- ISO
International Organisation for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- JMPR
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues (Joint Meeting on Pesticide Residues)
- LC
liquid chromatography
- LC–MS/MS
liquid chromatography with tandem mass spectrometry
- LOQ
limit of quantification
- Mo
Monitoring
- MoE
margin of exposure
- MRL
maximum residue level
- MS
Member States
- MS
mass spectrometry detector
- MS/MS
tandem mass spectrometry detector
- MW
molecular weight
- NEU
northern European Union
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant back interval
- PF
processing factor
- PHI
pre‐harvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- PROFile
(EFSA) Pesticide Residues Overview File
- RA
risk assessment
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SC
suspension concentrate
- SEU
southern European Union
- SL
soluble concentrate
- SMILES
simplified molecular‐input line‐entry system
- SP
water soluble powder
- STMR
supervised trials median residue
- TAR
total applied radioactivity
- TMDI
theoretical maximum daily intake
- UL
upper tolerable intake level
- UV
ultraviolet (detector)
- WHO
World Health Organization
Appendix A – Summary of authorised uses considered for the review of MRLs
A.1. Authorised post‐harvest uses in EU
| Crop | Member State | Pests controlled | Formulation | Application | Application rate | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | MS code | F, G/Ia | Typeb | Conc. a.s. | Method kind | Growth stage (BBCH range)c | Number (min–max) | Minimum interval (days) |
g a.s./hL min–max |
Water L/ha min–max |
Rate & Unit | WHP (days)d | Remarks | |
| Almonds | DE, IT | I | Insects as storage pest | GA | 998 g/kg | Post‐harvest treatment – gassing | 99 | 3 | – | – | 128 g a.i./m3 | 1 |
Max CTP 1,500 g h/m3 per treatment. Max CTP 4,500 g h/m3 per year. Application in case of infestation in containers (for consignments of goods) and indoor. |
|
| Brazil nuts | IT | I | Insects as storage pest | GA | 998 g/kg | Post‐harvest treatment – gassing | 99 | 3 | – | – | 128 g a.i./m3 | 1 | Max CTP 1,500 g h/m3 per treatment. | |
| Cashew nuts | IT | I | Insects as storage pest | GA | 998 g/kg | Post‐harvest treatment – gassing | 99 | 3 | – | – | 128 g a.i./m3 | 1 | Max CTP 1,500 g h/m3 per treatment. | |
| Chestnuts | DE | I | Insects as storage pest | GA | 998 g/kg | Post‐harvest treatment – gassing | 99 | 3 | – | – | 128 g a.i./m3 | 1 |
Max CTP 1,500 g h/m3 per treatment. Max CTP 4,500 g h/m3 per year. Application in case of infestation in containers (for consignments of goods) and indoor. |
|
| Hazelnuts | DE, IT | I | Insects as storage pest | GA | 999 g/kg | Post‐harvest treatment – gassing | 100 | 3 | – | – | 128 g a.i./m3 | 2 |
Max CTP 1,500 g h/m3 per treatment. Max CTP 4,500 g h/m3 per year. Application in case of infestation in containers (for consignments of goods) and indoor. |
|
| Macadamias | IT | I | Insects as storage pest | GA | 998 g/kg | Post‐harvest treatment – gassing | 99 | 3 | – | – | 128 g a.i./m3 | 1 | Max CTP 1,500 g h/m3 per treatment. | |
| Pecans | IT | I | Insects as storage pest | GA | 998 g/kg | Post‐harvest treatment – gassing | 99 | 3 | – | – | 128 g a.i./m3 | 1 | Max CTP 1,500 g h/m3 per treatment. | |
| Pine nut kernels | IT | I | Insects as storage pest | GA | 998 g/kg | Post‐harvest treatment – gassing | 99 | 3 | – | – | 128 g a.i./m3 | 1 | Max CTP 1,500 g h/m3 per treatment. | |
| Pistachios | IT | I | Insects as storage pest | GA | 998 g/kg | Post‐harvest treatment – gassing | 99 | 3 | – | – | 128 g a.i./m3 | 1 | Max CTP 1,500 g h/m3 per treatment. | |
| Walnuts | DE | I | Insects as storage pest | GA | 998 g/kg | Post‐harvest treatment – gassing | 99 | 3 | – | – | 128 g a.i./m3 | 1 |
Max CTP 1,500 g h/m3 per treatment. Max CTP 4,500 g h/m3 per year. Application in case of infestation in containers (for consignments of goods) and indoor. |
|
| Raisins | FR | I | Insects as storage pest | GA | 998 g/kg | Post‐harvest treatment – gassing | 99 | 2 | – | – | 128 g a.i./m3 | 6 |
Treatment of dried raisins only. max CTP 1500 g h/m3 per treatment.Only for treatments in room or fumigation container. |
|
| Cereals (emptied cereal mills, empty grain storage room) | NL | I | Insects as storage pest | GA | 998 g/kg | Post‐harvest treatment – gassing | 99 | 2 | – | – | 128 g a.i./m3 | 1 |
Max CTP 1,500 g h/m3 per treatment. Two treatments per year allowed emptied cereal grain mills and connected storage facilities and emptied cereal grain storage. The first 10 min of the flour and bran production after fumigation of a mill need to be collected. The collected material should not be used for human food or animal feed but must be destroyed. The production of the next 50 min needs to be collected and mixed during the next production process. All production lines of food processing facilities should be inspected, and any food residue should be collected. The collected food material should not be used for human food or animal feed but must be destroyed. |
|
| Cocoa beans | NL | I | Insects as storage pest | GA | 998 g/kg | Post‐harvest treatment – gassing | 99 | 2 | – | – | 128 g a.i./m3 | 1 |
Max CTP of 750 g h/m3 per treatment. The same batch of cocoa beans is treated only once. |
|
MS: Member State; a.s.: active substance; a.i.: active ingredient; CTP: concentration Time Product.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 6th Edition. Revised May 2008. Catalogue of pesticide.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
WHP: Withholding period.
Appendix B – List of end points
B.1. Residues in plants
B.1.1. Nature of residues and methods of analysis in plants
B.1.1.1. Metabolism studies, methods of analysis and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crop(s) | Application(s) | Sampling (DAT) | Comment/Source |
|---|---|---|---|---|---|
| Fruit crops | – | – | – | Studies investigating the nature of residues in primary crops were not available. Open literature papers on degradation of sulfuryl fluoride in several food commodities have been available during the peer review but considered not sufficient (United Kingdom, 2004, 2009; EFSA, 2010) (data gap) | |
| Root crops | – | – | – | ||
| Leafy crops | – | – | – | ||
| Cereals/grass | – | – | – | ||
| Pulses/oilseeds | – | – | – | ||
| Miscellaneous | – | – | – |
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) | Comment/Source |
|---|---|---|---|---|---|
| Root/tuber crops | – | – | – | Sulfuryl fluoride is authorised only as fumigation of the interior of buildings. Therefore, studies investigating the nature of sulfuryl fluoride on rotational crops are not required | |
| Leafy crops | – | – | – | ||
| Cereal (small grain) | – | – | – | ||
| Other | – | – | – |
| Processed commodities (hydrolysis study) | Conditions | Stable? | Comment/Source |
|---|---|---|---|
| Pasteurisation (20 min, 90°C, pH 4) | Inconclusive | No studies investigating the nature of residues of sulfuryl fluoride in processed commodities available in this review. Pending the outstanding data on the nature and magnitude of residues in treated commodities, further studies might be required | |
| Baking, brewing and boiling (60 min, 100°C, pH 5) | Inconclusive | ||
| Sterilisation (20 min, 120°C, pH 6) | Inconclusive | ||
| Other processing conditions | Inconclusive |
DAT: days after treatment; PBI: plant‐back interval; GC‐ECD: gas chromatography with electron capture detector; GC‐MS: gas chromatography with mass spectrometry; LOQ: limit of quantification; ILV: independent laboratory validation.

B.1.1.2. Stability of residues in plants
| Plant products (available studies) | Category | Commodity | T (°C) | Stability period | Compounds covered | Comment/Source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
| High oil content | Walnuts |
–20°C Ambient |
3 1 |
Months Month |
Fluoride ion |
EFSA, 2010 For sulfuryl fluoride data are not required as the samples of residue trials were analysed in most cases in less than 1 month from sampling |
|
| High starch content | Wheat grain |
–20°C Ambient |
3 1 |
Months Month |
Fluoride ion | ||
| Maize grain |
–20°C Ambient |
3 1 |
Months Month |
Fluoride ion | |||
| Processed products | Wheat flour |
–20°C Ambient |
3 1 |
Months Month |
Fluoride ion | ||
| Raisins |
–20°C Ambient |
3 1 |
Months Month |
Fluoride ion | |||
| Maize meal |
–20°C Ambient |
3 1 |
Months Month |
Fluoride ion | |||
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials – Primary crops – Sulfuryl fluoride
| Commodity | Region/Indoora | Residue levels observed in the supervised residue trials (mg/kg) | Comments/Source | Calculated MRL (mg/kg) | HRb (mg/kg) | STMRc (mg/kg) |
|---|---|---|---|---|---|---|
| RD‐Mo and RD‐RA1: sulfuryl fluoride | ||||||
| Almonds, cashew nuts, chestnuts, hazelnuts/cobnuts, Macadamias, pecans pine nut, pistachios walnuts | Post‐harvest |
Hazelnuts (without shells): 1.6; 1.8; 2; 2.1 |
Combined dataset on almonds, pecan, pistachios (small scale trials) and hazelnuts (large scale trials) compliant with GAP (Austria, 2019). Extrapolation to tree nuts is acceptable. Calculated MRL based on mean + 4 SD | 10 (tentative)e | 5.16 | 1.7 |
| Dry raisins | Post‐harvest | 0.02; 2 × 0.03 | Trials on raisins compliant with the GAP (Austria, 2019). Calculated MRL based on mean + 4 SD | 0.05 (tentative)e , f , g | 0.03 | 0.03 |
| Cereals (grain) (use on emptied cereal mills, empty grain storage rooms) | Post‐harvest | – | No residue trials for sulfuryl fluoride available. Considering that the treatment is done in empty stores and mills, a no residue situation is expected for sulfuryl fluoride in cereals (EFSA, 2010) | 0.01* (tentative)e | 0.01 | 0.01 |
| Cereals (milling products) (use on emptied cereal mills, empty grain storage rooms) | Post‐harvest | – | No residue trials analysing for sulfuryl fluoride are available. Considering that the treatment is performed in empty stores and mills, a no residue situation is expected for sulfuryl fluoride in cereals (EFSA, 2010) | 0.01* (tentative)e , f | 0.01 | 0.01 |
| Cocoa beans | Post‐harvest | 4 × < 0.004; 0.008; 0.013 | Results from one study with cocoa beans placed in six separate compartments of a fumigation chamber (Belgium, 2013). Calculated MRL based on mean + 4 SD | 0.03 (tentative)e , h | 0.013 | 0.01 |
MRL: maximum residue level; GAP: Good Agricultural Practice; SD: standard deviation.
Indicates that the MRL is proposed at the limit of quantification.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue.
Supervised trials median residue.
In the trials on pistachio, pecans and almonds, residues were reported in whole nuts and it is not clear whether the nuts were treated and analysed with or without the shell. Nevertheless, residue data on a wide number of tree nut species as reported by the RMS (Austria, 2019) indicated that residue levels in shelled and unshelled commodities do not differ to a significant extent suggesting that sulfuryl fluoride easily penetrates the outer lignified layer of the nuts. Therefore, the information on whether the treatment was done on shelled or unshelled nuts is not considered relevant and the available data are acceptable.
A tentative MRL was derived due to uncertainties on the nature of sulfuryl fluoride in plant commodities after the fumigation treatment.
Although MRLs are set only on raw agricultural commodities, EFSA considered the available trials to derive a tentative MRL for processed commodities (cereals milling products and raisins).
A tentative MRL is derived based on a reduced number of trials.
A tentative MRL is derived since available information do not allow to conclude whether results are from independent trials. Moreover, an analytical method for enforcement in cocoa beans is still missing.
B.1.2.2. Summary of residues data from the supervised residue trials – Primary crops – Fluoride ion
| Commodity | Region/Indoora | Residue levels observed in the supervised residue trials (mg/kg) | Comments/Source | Calculated MRL (mg/kg) | HRb (mg/kg) | STMRc (mg/kg) |
|---|---|---|---|---|---|---|
| RD‐Mo and RD‐RA2: fluoride ion | ||||||
|
Almonds, cashew nuts, chestnuts, hazelnuts/cobnuts Macadamias, pecans pine nut, pistachios walnuts |
Post‐harvest |
Hazelnuts (without shells): 10.6; 12.6; 15.7; 16.2 Residue levels in controls: Walnut: 6 × < 2.2 |
Combined data set on almonds, pecan, pistachios (small scale trials) and hazelnuts (large scale trials) compliant with GAP (Austria, 2019). Extrapolation to tree nuts is acceptable. Calculated MRL based on mean + 4 SD | 30 (tentative)e | 21 | 15.7 |
| Dry raisins | Post‐harvest | 2 × < 0.5; 0.58 |
Trials on raisins compliant with GAP (Austria, 2019) Calculated MRL based on mean + 4 SD |
0.8 (tentative)e , f , g | 0.58 | 0.50 |
| Cereals grain (use on emptied cereal mills, empty grain storage rooms) | Post‐harvest |
Residue levels in controls: Grain: 13 × < 2 |
Results from controls (Austria, 2019) were considered to derive a tentative MRL, assuming that an appropriate risk mitigation measure is in place | 2 (tentative)h | 2 | 2 |
| Cereals flour (use on emptied cereal mills, empty grain storage rooms) | Post‐harvest | Residue levels in controls: 6 × < 1; 12 × < 2 | Results from controls (Austria, 2019) were considered to derive a tentative MRL, assuming that an appropriate risk mitigation measure is in place | 2 (tentative)f , h | 2 | 2 |
| Cereals bran (use on emptied cereal mills, empty grain storage rooms) | Post‐harvest | Residue levels in controls: 6 × < 1; 4 × < 2; 3 × < 4 | Results from controls (Austria, 2019) were considered to derive a tentative MRL, assuming that an appropriate risk mitigation measure is in place | 4 (tentative)f , h | 4 | 4 |
| Wheat germ (use on emptied cereal mills, empty grain storage rooms) | Post‐harvest | Residue levels in controls: 7 × < 0.5; < 0.1 | Results from controls (Austria, 2019) were considered to derive a tentative MRL, assuming that an appropriate risk mitigation measure is in place | 1 (tentative)f , h | 1 | 1 |
| Maize starch (use on emptied cereal mills, empty grain storage rooms) | Post‐harvest | Residue levels in controls: 5 × < 0.5; 0.74; 0.75 | Results from controls (Austria, 2019) were considered to derive a tentative MRL, assuming that an appropriate risk mitigation measure is in place | 1 (tentative)f , h | 1 | 1 |
| Semolina, wheat middlings, maize meal, polished rice, maize oil and maize grits (use on emptied cereal mills, empty grain storage rooms) | Post‐harvest |
Residue levels in controls: Semolina: 3 × < 0.5 Wheat middlings: 3 × < 0.5 Polished rice: 10 × < 0.5 Maize meal: 10 × < 0.5 Maize oil: 7 × < 0.5 Maize grits: 7 × < 0.5 |
Results from controls (Austria, 2019) were considered to derive a tentative MRL, assuming that an appropriate risk mitigation measure is in place | 0.5 (tentative)f , h | 0.5 | 0.5 |
| Cereals, other milling products (use on emptied cereal mills, empty grain storage rooms) | Post‐harvest | – | No residue trials available | – | – | – |
| Cocoa beans | Post‐harvest | 3.72; 3.43; 3.44; 3.7; 3.76; 3.52 | Results from one study with cocoa beans placed in six separate compartments of a fumigation chamber (Belgium, 2013). Available information is not sufficient to conclude that results are independent. Calculated MRL based on mean + 4 SD | 5 (tentative)e , i) | 3.76 | 3.61 |
MRL: maximum residue level; GAP: Good Agricultural Practice; SD: standard deviation.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue.
Supervised trials median residue.
In the trials on pistachio, pecans and almonds, residues were reported in whole nuts and it is not clear whether the nuts were treated and analysed with or without the shell. Nevertheless, residue data on a wide number of tree nut species as reported by the RMS (Austria, 2019), indicated that residue levels in shelled and unshelled commodities do not differ to a significant extent suggesting that sulfuryl fluoride easily penetrates the outer lignified layer of the nuts. Therefore, the information on whether the treatment was done on shelled or unshelled nuts is not considered relevant and the available data are acceptable.
A tentative MRL was derived due to uncertainties on the nature of sulfuryl fluoride in plant commodities after the fumigation treatment.
Although MRLs are set only on raw agricultural commodities, EFSA considered the available trials to derive a tentative MRL for processed commodities (cereals milling products and raisins).
A tentative MRL is derived based on a reduced number of trials.
A tentative MRL was derived due to uncertainties on the nature of sulfuryl fluoride in plant commodities after the fumigation treatment and pending more detailed information on the background levels of fluoride ion.
A tentative MRL is derived since available information do not allow to conclude whether results are from independent trials. Moreover, an analytical method for enforcement in cocoa beans is still missing.
B.1.2.3. Residues in rotational crops

B.1.2.4. Processing factors
No processing factors are available, and they are not required. Nevertheless, according to the authorised uses reported during the MRL review, sulfuryl fluoride is authorised for fumigation on raisins and in empty stores and mills where cereals and milling products are produced and stored. Therefore, MRLs were derived for processed commodities as raisins and cereal milling products and reported in Appendices B.1.2.1 and B.1.2.2.
B.2. Residues in livestock
The authorised use is on empty stores and mills. Assuming that proper risk mitigation measures are currently in place, livestock are not expected to be exposed to residues of sulfuryl fluoride and fluoride ion above the LOQ or above the background levels. Therefore, there is no need to further investigate residues in livestock.
Regarding background concentrations from other sources than the authorised uses, according to the scientific opinion of the Panel on Dietetic Products, Nutrition and Allergies (NDA) (EFSA, 2013), products of animal origin may contain fluoride ion between 0.05 and 0.15 mg/kg in milk and dairy, 0.15–0.29 mg/kg in meat and meat products and 0.18 mg/kg in eggs. Based on these data, it is expected that MRLs of 0.2 mg/kg in milk and eggs and 0.3 mg/kg in livestock tissues will cover the background level of fluoride naturally occurring in these animal commodities. Nevertheless, these MRLs should be considered tentative only and should be confirmed by more detailed information on the background levels. Moreover, an analytical method for the enforcement of fluoride ion in animal commodities is not available.
B.3. Consumer risk assessment
B.3.1. Consumer risk assessment without consideration of the existing CXLs


B.3.2. Consumer risk assessment with consideration of the existing CXLs


Consumer exposure assessment through drinking water resulting from groundwater metabolite(s) according to SANCO/221/2000 rev.10 Final (25/02/2003).

B.4. Proposed MRLs
| Code number | Commodity | Existing EU MRL (mg/kg) | Existing CXLa (mg/kg) | Outcome of the review | |
|---|---|---|---|---|---|
| MRL (mg/kg) | Comment | ||||
| Enforcement residue definition 1: sulfuryl fluoride | |||||
| 120010 | Almonds | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| 120020 | Brazil nuts | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| 120030 | Cashew nuts | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| 120040 | Chestnuts | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| 120050 | Coconuts | 10 | 3 | 3 |
Further consideration neededc Data gaps #1, 2 |
| 120060 | Hazelnuts | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| 120070 | Macadamia | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| 120080 | Pecans | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| 120090 | Pine nuts | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| 120100 | Pistachios | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| 120110 | Walnuts | 10 | 3 | 10 |
Further consideration neededb Data gaps #1, 2 |
| – | Raisins | – | 0.06 | 0.06 |
Further consideration neededd Data gaps #1, 2 |
| 500000 | Cereals grain | 0.05 | 0.05 | 0.01* |
Further consideration needede Data gaps #1, 2 |
| – | Cereals, milling products | – | 0.1 | 0.01* |
Further consideration needede Data gaps #1, 2 |
| 640000 | Cocoa (fermented beans) | 0.02* | – | 0.03 |
Further consideration neededf Data gaps #1, 2, 3, 4 |
| – | Other commodities of plant and/or animal origin | See Reg. 839/2008 | – | – | Further consideration neededg |
| Enforcement residue definition 2: fluoride ion | |||||
| 110000 | Citrus fruits | – | 0.2 |
Further consideration neededh Data gap #5, 7 |
|
| 120010 | Almonds | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| 120020 | Brazil nuts | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| 120030 | Cashew nuts | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| 120040 | Chestnuts | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| 120050 | Coconuts | 25 | 15 | 15 |
Further consideration neededc Data gaps #1, 2 |
| 120060 | Hazelnuts | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| 120070 | Macadamia | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| 120080 | Pecans | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| 120090 | Pine nuts | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| 120100 | Pistachios | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| 120110 | Walnuts | 25 | 15 | 30 |
Further consideration neededb Data gaps #1, 2 |
| – | Raisins | – | 3 | 3 |
Further consideration neededd Data gaps #1, 2 |
| 130000 | Pome fruits | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 140000 | Stone fruits | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 150000 | Berries and small fruits | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 161010 | Dates | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 161020 | Figs | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 161030 | Table olives | 2* | 0.2 |
Further consideration neededh Data gap #5 |
|
| 161040 | Kumquats | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 161050 | Carambolas | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 161060 | Kaki/Japanese persimmons | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 161070 | Jambuls/jambolans | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 161040 | Kumquats | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 162000 | Miscellaneous fruits (inedible peel, small) | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163010 | Avocados | 2* | 0.2 |
Further consideration neededh Data gap #5 |
|
| 163020 | Bananas | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163030 | Mangoes | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163040 | Papayas | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163050 | Granate apples/pomegranates | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163060 | Cherimoyas | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163070 | Guavas | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163080 | Pineapples | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163090 | Breadfruits | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163100 | Durians | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 163110 | Soursops/guanabanas | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 200000 | Vegetables, fresh or frozen | 2* | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 300000 | Pulses | 2* | – | 2 |
Further consideration neededi) Data gap #5 |
| 400000 | Oilseeds and oil fruits | 2* | – | 2 |
Further consideration neededi) Data gap #5 |
| 500000 | Cereals grain | 2* | 25 | 2 |
Further consideration needede Data gaps #1, 2, 5, 6 |
| – | Cereals flour | – | 80 | 2 |
Further consideration needede Data gaps #1, 2, 5, 6 |
| – | Cereals bran | – | 150 | 4 |
Further consideration needede Data gaps #1, 2, 5, 6 |
| – | Wheat germ | – | 150 | 1 |
Further consideration needede Data gaps #1, 2, 5, 6 |
| – | Maize Starch | – | – | 1 |
Further consideration neededf Data gaps #1, 2, 5, 6 |
| – |
Semolina Wheat middlings Maize grits Maize oil |
– | – | 0.5 |
Further consideration neededf Data gaps #1, 2, 5, 6 |
| – |
Maize meal Polished rice |
– | 150 | 0.5 |
Further consideration needede Data gaps #1, 2, 5, 6 |
| 610000 | Tea (dried leaves of Camellia sinensis) | 350 | – | 400 |
Further consideration neededh Data gap #5, 7 |
| 620000 | Coffee beans | 5 | – | 5 |
Further consideration neededi) Data gap #5, 7 |
| 630000 | Herbal infusions | 10 | – | 2 |
Further consideration neededh Data gap #5, 7 |
| 640000 | Cocoa (fermented beans) | 10 | – | 5 |
Further consideration neededf Data gaps #1, 2, 3, 4 |
| 650000 | Carobs | 10 | – | 10 |
Further consideration neededi) Data gap #5, 7 |
| 700000 | Hops | 10 | – | 10 |
Further consideration neededi) Data gap #5, 7 |
| 800000 | Spices | 5 | – | 5 |
Further consideration neededi) Data gap #5, 7 |
| 900000 | Sugar plants | 2 | – | 2 |
Further consideration neededi) Data gap #5, 7 |
| 1010000 | Products of animal origin, tissues | 1 | – | 0.3 |
Further consideration neededh Data gaps #5, 7 |
| 1020000 | Milks | 0.2 | – | 0.2 |
Further consideration neededh Data gaps #5, 7 |
| 1030000 | Eggs | 0.2 | – | 0.2 |
Further consideration needed h Data gaps #5, 7 |
MRL: maximum residue level; CXL: codex maximum residue limit.
Indicates that the MRL is set at the limit of quantification.
The CAC adopted CXLs for the residue definition sulfuryl fluoride only. Although no specific CXLs are set for the fluoride ion, both sulfuryl fluoride and fluoride ion were analysed in the trials considered by the JMPR to derive the CXLs. In order to support risk managers, EFSA considered the data from the JMPR to calculate the corresponding MRL proposals for fluoride ion for commodities for which CXLs are established.
The proposed MRL is based on a GAP evaluated at EU level which is not fully supported by data. Although no risk for consumers has been identified for sulfuryl fluoride, lacking information on the toxicological reference values for fluoride ion, an overall consumer risk assessment could not be performed. The calculation of the margin of exposure indicated that the contribution from the existing EU use on this commodity to the overall fluoride exposure is low. The existing CXL is covered by the proposed MRL.
The proposed MRL is based on the existing CXL which is not fully supported by data. Although no risk for consumers has been identified for sulfuryl fluoride, lacking information on the toxicological reference values for fluoride ion, an overall consumer risk assessment could not be performed. The calculation of the margin of exposure indicated that the contribution from the existing CXL on this commodity to the overall fluoride exposure is low. There are no relevant authorisations or import tolerances reported at EU level.
The proposed MRL is based on the existing CXL which is not fully supported by data. Although no risk for consumers has been identified for sulfuryl fluoride, lacking information on the toxicological reference values for fluoride ion, an overall consumer risk assessment could not be performed. The calculation of the margin of exposure indicated that the contribution from the existing CXL on this commodity to the overall fluoride exposure is low. The GAP authorised at EU level lead to a lower MRL.
The proposed MRL is based on a GAP evaluated at EU level which is not fully supported by data. Although no risk for consumers has been identified for sulfuryl fluoride, lacking information on the toxicological reference values for fluoride ion, an overall consumer risk assessment could not be performed. The calculation of the margin of exposure indicated that the contribution from the existing EU use on this commodity to the overall fluoride exposure is low. The existing CXL is higher but its contribution to the overall fluoride exposure is higher.
The proposed MRL is based on a GAP evaluated at EU level which is not fully supported by data. Although no risk for consumers has been identified for sulfuryl fluoride, lacking information on the toxicological reference values for fluoride ion, an overall consumer risk assessment could not be performed. The calculation of the margin of exposure indicated that the contribution from the existing EU use on this commodity to the overall fluoride exposure is low. No CXL is available.
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered.
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. As fluoride ion is naturally occurring, a tentative MRL is proposed at background levels.
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. Lacking information on the background levels, the existing MRL is indicatively proposed.
Appendix C – Pesticide Residue Intake Model (PRIMo)
C.1. PRIMo(EU) – Sulfuryl fluoride


C.2. Acute PRIMo(EU) – Fluoride



C.3. Chronic PRIMo(scenario 1 background only) – Fluoride

C.4. Chronic PRIMo(scenario 2 background & uses) – Fluoride

C.5. PRIMo(CX) – Sulfuryl fluoride

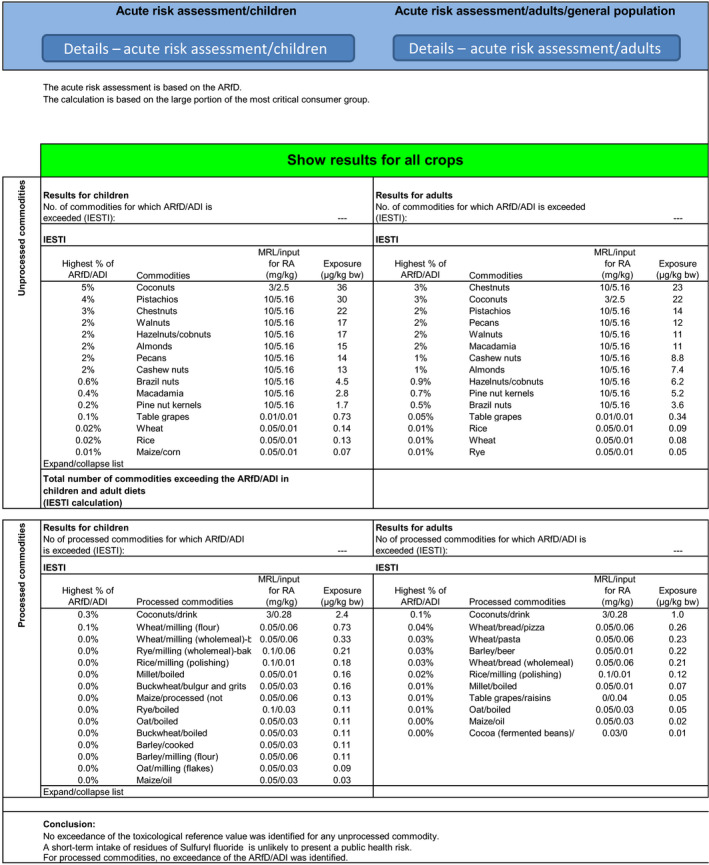
C.6. Acute PRIMo(CX) – Fluoride


C.7. Chronic PRIMo(scenario 3 background & uses & CXL) – Fluoride

C.8. Chronic PRIMo(scenario 4 background & uses & CXL Refined) – Fluoride

Appendix D – Input values for the exposure calculations
D.1. Consumer risk assessment without consideration of the existing CXLs
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition 1: sulfuryl fluoride | ||||
| Almonds | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Brazil nuts | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Cashew nuts | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Chestnuts | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Hazelnuts/cobnuts | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Macadamia | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Pecans | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Pine nut kernels | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Pistachios | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Walnuts | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Table grapes | 0.01 | STMR raisins (tentative)a/dehydration factor (4.7) | – | – |
| Raisins | – | See table grapes | 0.03 | HR (tentative) |
| Cereals (grain) | 0.01* | STMR cereal flour (tentative) a, b | 0.01* | HR (tentative) |
| Cereals (processed commodities) | – | See cereal grain | 0.01* | HR (tentative) |
| Cocoa beans | 0.01 | STMR (tentative) | 0.01 | HR (tentative) |
|
Risk assessment residue definition 2: fluoride ion SCENARIO 1 (background exposure based only on the background levels of fluoride) | ||||
| Citrus fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Tree nuts | 2.2 | Results from control samples (tentative, Austria, 2019) | – | – |
| Pome fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Stone fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Table grapes | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Wine grapes | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Strawberries | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Cane fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Small fruit & berries | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Miscellaneous fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Vegetables, fresh or frozen | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Pulses | 2 | EU MRL | – | – |
| Oilseeds and oil fruits | 2 | EU MRL | – | – |
| Cereal (grain) | 2 | Results from control samples (tentative, Austria, 2019) | – | – |
| Tea (dried leaves of Camellia sinensis) | 400 | Background level (tentative, EFSA, 2013) | – | – |
| Coffee beans | 5 | EU MRL | ||
| Herbal infusions | 2 | Background level (tentative, EFSA, 2013) | – | – |
| Cocoa beans | – | –c | – | – |
| Carobs | 10 | EU MRL | – | – |
| Hops | 10 | EU MRL | – | – |
| Spices | 5 | EU MRL | – | – |
| Sugar plants | 2 | EU MRL | – | – |
| Products of animal origin, except milks and eggs | 0.29 | Background level (tentative, EFSA, 2013) | – | – |
| Milks | 0.15 | Background level (tentative, EFSA, 2013) | – | – |
| Eggs | 0.18 | Background level (tentative, EFSA, 2013) | – | – |
|
Risk assessment residue definition 2: fluoride ion SCENARIO 2 (background exposure plus exposure resulting from fumigation with sulfuryl fluoride according to the GAPs reported in Appendix A) | ||||
| Citrus fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Tree nuts, except coconuts | 15.7 | STMR (tentative) | 21 | HR (tentative) |
| Coconuts | 2.2 | Results from control samples (tentative, Austria, 2019) | – | – |
| Pome fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Stone fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Table grapes | 0.2 | Background level (tentative, EFSA, 2013)d | – | – |
| Raisins | – | See table grapes | 0.58 | HR (tentative) |
| Wine grapes | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Strawberries | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Cane fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Small fruit & berries | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Miscellaneous fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Vegetables, fresh or frozen | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Pulses | 2 | EU MRL | – | – |
| Oilseeds and oil fruits | 2 | EU MRL | – | – |
| Cereal (grain) | 2 | STMR cereal flour (tentative)a , b | 2 | HR cereals grain (tentative) |
| Barley and wheat/milling (flour) | – | See cereal grain | 2 | HR cereals flour (tentative) |
| Barley/beer | – | See cereal grain | 0.4 | HR cereals grain × PF (0.2)e (tentative) |
| Barley/cooked | – | See cereal grain | 2 | HR cereals grain (tentative) |
| Buckwheat/bulgur and grits | – | See cereal grain | 2 | HR cereals grain (tentative) |
| Buckwheat/boiled | – | See cereal grain | 2 | HR cereals grain (tentative) |
| Maize/oil | – | See cereal grain | 0.5 | HR maize oil (tentative) |
| Maize/processed (not specified) | – | See cereal grain | 2 | HR cereals flour (tentative) |
| Millet/boiled | – | See cereal grain | 0.8 | HR cereals grain × PF (0.4) f (tentative) |
| Oat/milling (flakes) | – | See cereal grain | 2 | HR cereals grain (tentative) |
| Oat/boiled | – | See cereal grain | 2 | HR cereals grain (tentative) |
| Rice/milling (polishing) | – | See cereal grain | 0.5 | HR polished rice (tentative) |
| Rye/milling (wholemeal)‐baking | – | See cereal grain | 2 | HR cereals flour (tentative) |
| Rye/boiled | – | See cereal grain | 2 | HR cereals grain (tentative) |
| Wheat/bread (wholemeal) | – | See cereal grain | 2 | HR cereals flour (tentative) |
| Wheat/bread/pizza | – | See cereal grain | 2 | HR cereals flour (tentative) |
| Wheat/pasta | – | See cereal grain | 2 | HR cereals flour (tentative) |
| Wheat/milling (wholemeal)‐baking | – | See cereal grain | 2 | HR cereals flour (tentative) |
| Tea (dried leaves of Camellia sinensis) | 400 | Background level (tentative, EFSA, 2013) | – | – |
| Coffee beans | 5 | EU MRL | – | – |
| Herbal infusions | 2 | Background level (tentative, EFSA, 2013) | – | – |
| Cocoa beans | 3.61 | STMR (tentative) | 3.76 | HR (tentative) |
| Carobs | 10 | EU MRL | – | – |
| Hops | 10 | EU MRL | – | – |
| Spices | 5 | EU MRL | – | – |
| Sugar plants | 2 | EU MRL | – | – |
| Products of animal origin, tissues | 0.29 | Background level (tentative, EFSA, 2013) | – | – |
| Milks | 0.15 | Background level (tentative, EFSA, 2013) | – | – |
| Eggs | 0.18 | Background level (tentative, EFSA, 2013) | – | – |
STMR: supervised trials median residue; HR: highest residue; PF: processing factor.
Indicates that the input value is proposed at the limit of quantification.
Lacking detailed consumption data for processed products to be used for chronic exposure assessments, the STMR values for processed raisins and cereals milling products were combined with the consumption data for unprocessed grapes and cereals. For grapes, a dehydration factor of 4.7 was considered to recalculate the values from dried raisins to fresh grapes.
Considering that most of the consumed cereal products are derived from a milled product which is then further processed, STMR from cereal flour was used as input value for the chronic assessment of all processed commodities based on cereals.
Lacking information on the background levels and results from controls, cocoa beans were not considered in the exposure calculation.
Background levels were used as input value as STMR from trials (0.5)/dehydration factor (4.7) result in a lower input value (0.1).
A default PF of 0.2 was applied considering the dilution of the residues expected for the preparation of the beer.
A default PF of 0.4 was applied to recalculate the large portion expressed as processed product to the unprocessed commodity.
D.2. Consumer risk assessment with consideration of the existing CXLs
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition 1: sulfuryl fluoride | ||||
| Almonds | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Brazil nuts | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Cashew nuts | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Chestnuts | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Coconuts | 0.28 | STMR (CXL, tentative) | 2.5 | HR (CXL, tentative) |
| Hazelnuts/cobnuts | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Macadamia | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Pecans | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Pine nut kernels | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Pistachios | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Walnuts | 1.7 | STMR (tentative) | 5.16 | HR (tentative) |
| Table grapes | 0.01 | STMR raisins (CXL, tentative)a/dehydration factor (4.7) | – | – |
| Raisins | – | See table grapes | 0.04 | HR (CXL, tentative) |
| Cereal (grain) | 0.01 | STMR flour (CXL, tentative)a , b | 0.03 | HR cereal grain (CXL, tentative) |
| Barley and wheat/milling (flour) | – | See cereal grain | 0.06 | HR wheat flour (CXL, tentative) |
| Barley/beer | – | See cereal grain | 0.01 | HR cereal grain × PF (0.2)c (CXL, tentative) |
| Barley/cooked | – | See cereal grain | 0.03 | HR cereal grain (CXL, tentative) |
| Buckwheat/bulgur and grits | – | See cereal grain | 0.03 | HR cereal grain (CXL, tentative) |
| Buckwheat/boiled | – | See cereal grain | 0.03 | HR cereal grain (CXL, tentative) |
| Maize/oil | – | See cereal grain | 0.03 | HR cereal grain (CXL, tentative)d |
| Maize/processed (not specified) | – | See cereal grain | 0.06 | HR wheat flour (CXL, tentative) |
| Millet/boiled | – | See cereal grain | 0.01 | HR cereal grain × PF (0.4)e (CXL, tentative) |
| Oat/milling (flakes) | – | See cereal grain | 0.03 | HR cereal grain (CXL, tentative) |
| Oat/boiled | – | See cereal grain | 0.03 | HR cereal grain (CXL, tentative) |
| Rice/milling (polishing) | – | See cereal grain | 0.01 | HR cereal grain × PF (0.4)e (CXL, tentative) |
| Rye/milling (wholemeal)‐baking | – | See cereal grain | 0.06 | HR wheat flour (CXL, tentative) |
| Rye/boiled | – | See cereal grain | 0.03 | HR cereal grain (CXL, tentative) |
| Wheat/bread (wholemeal) | – | See cereal grain | 0.06 | HR wheat flour (CXL, tentative) |
| Wheat/bread/pizza | – | See cereal grain | 0.06 | HR wheat flour (CXL, tentative) |
| Wheat/pasta | – | See cereal grain | 0.06 | HR wheat flour (CXL, tentative) |
| Wheat/milling (wholemeal)‐baking | – | See cereal grain | 0.06 | HR wheat flour (CXL, tentative) |
| Cocoa beans | 0.01 | STMR (tentative) | 0.01 | HR (tentative) |
|
Risk assessment residue definition 2: fluoride ion SCENARIO 3 (background exposure plus exposure resulting from fumigation with sulfuryl fluoride according to the GAPs reported in Appendix A and according to all uses assessed by the JMPR) | ||||
| Citrus fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Tree nuts, except coconuts | 15.7 | STMR (tentative) | 21 | HR (tentative) |
| Coconuts | 2.4 | STMR (CXL, tentative) | 9.1 | HR (CXL, tentative) |
| Pome fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Stone fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Table grapes | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Table grapes (Raisins) | 0.5 | STMR raisins (CXL, tentative)a/dehydration factor (4.7) | 2.4 | HR raisins (CXL, tentative) |
| Wine grapes | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Strawberries | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Cane fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Small fruit & berries | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Miscellaneous fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Vegetables, fresh or frozen | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Pulses | 2 | EU MRL | – | – |
| Oilseeds and oil fruits | 2 | EU MRL | – | – |
| Cereal (grain) | 35 | STMR for wheat flour (CXL, tentative)(a),(b) | 21 | HR cereal grain (CXL, tentative) |
| Barley and wheat/milling (flour) | – | See cereal grain | 55 | HR wheat flour (CXL, tentative) |
| Barley/beer | – | See cereal grain | 4.2 | HR cereal grain × PF (0.2)c (CXL, tentative) |
| Barley/cooked | – | See cereal grain | 21 | HR cereal grain (CXL, tentative) |
| Buckwheat/bulgur and grits | – | See cereal grain | 21 | HR cereal grain (CXL, tentative) |
| Buckwheat/boiled | – | See cereal grain | 21 | HR cereal grain (CXL, tentative) |
| Maize/oil | – | See cereal grain | 21 | HR cereal grain (CXL, tentative)d |
| Maize/processed (not specified) | – | See cereal grain | 70 | HR maize flour (CXL, tentative) |
| Millet/boiled | – | See cereal grain | 8.4 | HR cereal grain × PF (0.4)e (CXL, tentative) |
| Oat/milling (flakes) | – | See cereal grain | 21 | HR cereal grain (CXL, tentative) |
| Oat/boiled | – | See cereal grain | 21 | HR cereal grain (CXL, tentative) |
| Rice/milling (polishing) | – | See cereal grain | 8.4 | HR cereal grain × PF (0.4)e (CXL, tentative) |
| Rye/milling (wholemeal)‐baking | – | See cereal grain | 55 | HR wheat flour (CXL, tentative) |
| Rye/boiled | – | See cereal grain | 21 | HR cereal grain (CXL, tentative) |
| Wheat/bread (wholemeal) | – | See cereal grain | 55 | HR wheat flour (CXL, tentative) |
| Wheat/bread/pizza | – | See cereal grain | 55 | HR wheat flour (CXL, tentative) |
| Wheat/pasta | – | See cereal grain | 55 | HR wheat flour (CXL, tentative) |
| Wheat/milling (wholemeal)‐baking | – | See cereal grain | 55 | HR wheat flour (CXL, tentative) |
| Tea (dried leaves of Camellia sinensis) | 400 | Background level (tentative, EFSA, 2013) | – | – |
| Coffee beans | 5 | EU MRL | – | – |
| Herbal infusions | 2 | Background level (tentative, EFSA, 2013) | – | – |
| Cocoa beans | 3.61 | STMR (tentative) | 3.76 | HR (tentative) |
| Carobs | 10 | EU MRL | – | – |
| Hops | 10 | EU MRL | – | – |
| Spices | 5 | EU MRL | – | – |
| Sugar plants | 2 | EU MRL | – | – |
| Products of animal origin, except milks and eggs | 0.29 | Background level (tentative, EFSA, 2013) | – | – |
| Milks | 0.15 | Background level (tentative, EFSA, 2013) | – | – |
| Eggs | 0.18 | Background level (tentative, EFSA, 2013) | – | – |
|
Risk assessment residue definition 2: fluoride ion SCENARIO 4 (background exposure plus exposure resulting from fumigation with sulfuryl fluoride according to the GAPs reported in Appendix A and according to the uses on coconuts and raisins assessed by the JMPR, excluding JMPR uses on cereals) | ||||
| Citrus fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Tree nuts, except coconuts | 15.7 | STMR (tentative) | – | – |
| Coconuts | 2.4 | STMR (CXL, tentative) | – | – |
| Pome fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Stone fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Table grapes | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Table grapes (Raisins) | 0.5 | STMR raisins (CXL, tentative)a/dehydration factor (4.7) | – | – |
| Wine grapes | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Strawberries | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Cane fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Small fruit & berries | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Miscellaneous fruits | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Vegetables, fresh or frozen | 0.2 | Background level (tentative, EFSA, 2013) | – | – |
| Pulses | 2 | EU MRL | – | – |
| Oilseeds and oil fruits | 2 | EU MRL | – | – |
| Cereal (grain) | 2 | STMR cereal flour (tentative)a , b | – | – |
| Barley and wheat/milling (flour) | – | See cereal grain | – | – |
| Barley/beer | – | See cereal grain | – | – |
| Barley/cooked | – | See cereal grain | – | – |
| Buckwheat/bulgur and grits | – | See cereal grain | – | – |
| Buckwheat/boiled | – | See cereal grain | – | – |
| Maize/oil | – | See cereal grain | – | – |
| Maize/processed (not specified) | – | See cereal grain | – | – |
| Millet/boiled | – | See cereal grain | – | – |
| Oat/milling (flakes) | – | See cereal grain | – | – |
| Oat/boiled | – | See cereal grain | – | – |
| Rice/milling (polishing) | – | See cereal grain | – | – |
| Rye/milling (wholemeal)‐baking | – | See cereal grain | – | – |
| Rye/boiled | – | See cereal grain | – | – |
| Wheat/bread (wholemeal) | – | See cereal grain | – | – |
| Wheat/bread/pizza | – | See cereal grain | – | – |
| Wheat/pasta | – | See cereal grain | – | – |
| Wheat/milling (wholemeal)‐baking | – | See cereal grain | – | – |
| Tea (dried leaves of Camellia sinensis) | 400 | Background level (tentative, EFSA, 2013) | – | – |
| Coffee beans | 5 | EU MRL | – | – |
| Herbal infusions | 2 | Background level (tentative, EFSA, 2013) | – | – |
| Cocoa beans | 3.61 | STMR (tentative) | – | – |
| Carobs | 10 | EU MRL | – | – |
| Hops | 10 | EU MRL | – | – |
| Spices | 5 | EU MRL | – | – |
| Sugar plants | 2 | EU MRL | – | – |
| Products of animal origin, except milks and eggs | 0.29 | Background level (tentative, EFSA, 2013) | – | – |
| Milks | 0.15 | Background level (tentative, EFSA, 2013) | – | – |
| Eggs | 0.18 | Background level (tentative, EFSA, 2013) | – | – |
STMR: supervised trials median residue; HR: highest residue; PF: processing factor; CXL: Codex maximum residue limit.
Indicates that the input value is proposed at the limit of quantification.
Lacking detailed consumption data for processed products to be used for chronic exposure assessments, the STMR values for processed raisins and cereals milling products were combined with the consumption data for unprocessed grapes and cereals. For grapes, a dehydration factor of 4.7 was considered to recalculate the values from dried raisins to fresh grapes.
Considering that most of the consumed cereal products are derived from a milled product which is then further processed, STMR from cereal flour was used as input value for the chronic assessment of all processed commodities based on cereals.
A default PF of 0.2 was applied considering the dilution of the residues expected for the preparation of the beer.
A default PF was not applied as, according to the available studies, no concentration of the residues is expected in oil.
A default PF of 0.4 was applied to recalculate the large portion expressed as processed product to the unprocessed commodity.
Appendix E – Decision tree for deriving MRL recommendations
1.


Appendix F – Used compound codes
1.
| Code/trivial namea | Chemical name/SMILES notation/InChiKeyb | Structural formulac |
|---|---|---|
| Sulfuryl fluoride |
Sulfuryl difluoride FS(F)(=O)=O OBTWBSRJZRCYQV‐UHFFFAOYSA‐N |

|
|
Fluoride ion (fluoride) |
Fluoride [F‐] KRHYYFGTRYWZRS‐UHFFFAOYSA‐M |
F− |
The name in bold is the name used in the conclusion.
ACD/Name 2019.1.1 ACD/Labs 2019 Release (File version N05E41, Build 110555, 18 July 2019).
ACD/ChemSketch 2019.1.1 ACD/Labs 2019 Release (File version C05H41, Build 110712, 24 July 2019).
Suggested citation: EFSA (European Food Safety Authority) , Anastassiadou M, Bernasconi G, Brancato A, Carrasco Cabrera L, Ferreira L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Nave S, Pedersen R, Reich H, Rojas A, Sacchi A, Santos M, Scarlato AP, Theobald A, Vagenende B and Verani A, 2021. Reasoned opinion on the review of the existing maximum residue levels for sulfuryl fluoride according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2021;19(1):6390, 72 pp. 10.2903/j.efsa.2021.6390
Requestor: European Commission
Question number: EFSA‐Q‐2010‐01080
Acknowledgement: EFSA wishes to thank the following EFSA staff members Chris Anagnostopoulos, Stathis Anagnos, Laszlo Bura, Viktorija Krivova, Silvia Ruocco and Viktor Toth for the support provided and the rapporteur Member State, Austria, for the preparatory work on this scientific output.
Approved: 18 December 2020
Notes
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, p. 1–32. Repealed by Regulation (EC) No 1107/2009.
Commission Directive 2010/38/EU of 18 June 2010 amending Council Directive 91/414/EEC to include sulfuryl fluoride as active substance. OJ No L 154, 19.6.2010, p. 21–23.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) No 540/2011 of 25 May 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 1–186.
Commission Implementing Regulation (EU) No 541/2011 of 1 June 2011 amending Implementing Regulation (EU) No 540/2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 187–188.
Commission Implementing Regulation (EU) 2017/270 of 16 February 2017 amending Implementing Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance sulfuryl fluoride. OJ L 40, 17.2.2017, p. 48–50.
The United Kingdom withdrew from EU on 1 February 2020. In accordance with the Agreement on the Withdrawal of the United Kingdom from the EU, and with the established transition period, the EU requirements on data reporting also apply to the United Kingdom data collected until 31 December 2020.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
Cocoa beans were not covered by this calculation since information on background levels and residue data from control samples were not available.
References
- Austria , 2019. Evaluation report prepared under Article 12.1 of Regulation (EC) No 396/2005. Review of the existing MRLs for sulfuryl fluoride, 12 December 2019 revised on March 2020. Available online: www.efsa.europa.eu
- Belgium , 2013. Registration report Part B. Section 4: Metabolism and Residues. Detailed summary of the risk assessment. Product code: ProFume. Active Substance: >998 g/kg sulfuryl fluoride. One Zone (post‐harvest) Zonal Rapporteur Member State: Belgium. December 2013.
- EFSA (European Food Safety Authority), 2005. Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission related to the Tolerable Upper Intake Level of Fluoride. 66 pp. 10.2903/j.efsa.2005.192 [DOI]
- EFSA (European Food Safety Authority), 2010. Conclusion on the peer review of the pesticide risk assessment of the active substance sulfuryl fluoride. EFSA Journal 2010;8(1):1441, 66 pp. 10.2903/j.efsa.2010.1441 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. Modification of the existing MRLs for sulfuryl fluoride and fluoride ion in chestnuts. EFSA Journal 2012;10(2):2591, 23 pp. 10.2903/j.efsa.2012.2591 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Technical report on the outcome of the consultation with Member States, the applicant and EFSA on the pesticide risk assessment for sulfuryl fluoride in light of confirmatory data. EFSA supporting publication 2015;EN‐870, 14 pp. 10.2903/j.efsa.2015.870 [DOI]
- EFSA (European Food Safety Authority), Brancato A, Brocca D, Ferreira L, Greco L, Jarrah S, Leuschner R, Medina P, Miron I, Nougadere A, Pedersen R, Reich H, Santos M, Stanek A, Tarazona J, Theobald A and Villamar‐Bouza L, 2018. Guidance on use of EFSA Pesticide Residue Intake Model (EFSA PRIMo revision 3). EFSA Journal 2018;16(1):5147, 43 pp. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2019. Pesticide Residue Intake Model‐ EFSA PRIMo revision 3.1. EFSA supporting publication 2019;16(3):EN‐1605, 15 pp. 10.2903/sp.efsa.2019.EN-1605 [DOI] [PMC free article] [PubMed]
- EFSA (European Food Safety Authority), 2020a. Completeness check report on the review of the existing MRLs of sulfuryl fluoride prepared by EFSA in the framework of Article 12 of Regulation (EC) No 396/2005, 9 March 2020. Available online: www.efsa.europa.eu
- EFSA (European Food Safety Authority), 2020b. Member States consultation report on the review of the existing MRLs of sulfuryl fluoride prepared by EFSA in the framework of Article 12 of Regulation (EC) No 396/2005, 10 November 2020 (first consultation). Available online: www.efsa.europa.eu
- EFSA (European Food Safety Authority), 2020c. Member States consultation report on the review of the existing MRLs of sulfuryl fluoride prepared by EFSA in the framework of Article 12 of Regulation (EC) No 396/2005, 9 December 2020 (second consultation). Available online: www.efsa.europa.eu
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2013. Scientific opinion on Dietary Reference Values for fluoride. EFSA Journal 2013;11(8):3332, 46 pp. 10.2903/j.efsa.2013.3332 [DOI] [Google Scholar]
- EURLs (European Union Reference Laboratories for Pesticide Residues), 2019. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Analytical methods validated by the EURLs and overall capability of official laboratories to be considered for the review of the existing MRLs for sulfuryl fluoride. September 2019. Available online: www.efsa.europa.eu
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/IV/95‐rev., 22 July 1996.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev. 6, 22 July 1997.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev. 2, 22 July 1997.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev. 3, 22 July 1997.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals.7039/VI/95 22 July 1997. As amended by the document: classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirement for Annex II (part A, section 4) and Annex III (part A, section 5 of Directive 91/414. SANCO/3029/99‐rev. 4.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010-rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2016. Review report for the active substance sulfuryl fluoride. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 11 May 2010 in view of the inclusion of sulfuryl fluoride in Annex I of Directive 91/414/EEC. SANCO/10567/2010 ‐ Rev 1, 7 December 2016.
- European Commission , 2017. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev.10.3, June 2017.
- FAO (Food and Agriculture Organization of the United Nations), 2005. Sulfuryl fluoride. In: Pesticide residues in food – 2005. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues. FAO Plant Production and Protection Paper 183.
- FAO (Food and Agriculture Organization of the United Nations), 2009. Submission and evaluation of pesticide residues data for the estimation of Maximum Residue Levels in food and feed. Pesticide Residues. 2nd Edition. FAO Plant Production and Protection Paper 197, 264 pp.
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org
- OECD (Organisation for Economic Co‐operation and Development), 2013. Guidance document on residues in livestock. In: Series on Pesticides No 73. ENV/JM/MONO(2013)8, 04 September 2013.
- United Kingdom , 2004. Draft Assessment Report (DAR) on the active substance sulfuryl fluoride prepared by the rapporteur Member State the United Kingdom in the framework of Directive 91/414/EEC, October 2004.
- United Kingdom , 2009. Final Addendum to the Draft Assessment Report on sulfuryl fluoride, compiled by EFSA, January 2009.
- United Kingdom , 2015. Addendum to the assessment report on sulfuryl fluoride, confirmatory data, June 2015. Available online: www.efsa.europa.eu.
- WHO , 2006. Guidelines for drinking‐water quality ‐ 2nd ed Contents v.2 Health criteria and other supporting information 1. Drinking water‐standards ISBN 92 4 154480 5 (v 2) (NLM Classification WA 675) summarised in WHO Fluoride in drinking water report 2006.


