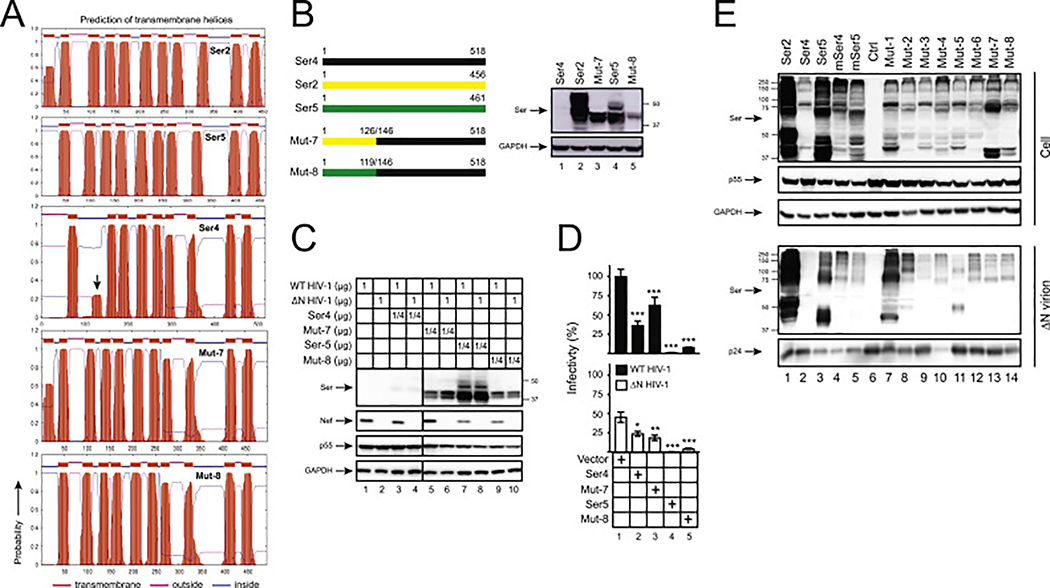
Fig. 6. Analysis of Ser2-Ser4 and Ser5-Ser4 chimeric protein expression and anti-HIV-1 activity.
(A) Ser2, Ser4, Ser5, and their chimeric protein topology were predicted by TMHMM and are presented. The putative human Ser4 2nd TD domain is indicated by an arrow-head.
(B) Mut-7 and Mut-8 were created by swapping the indicated human Ser4 (black) region with that from human Ser2 (yellow) or Ser5 (green), respectively, and expressed from the pCMV6 vector that expresses a C-terminal FLAG tag. 293T cells were transfected with 1 μg vectors expressing WT and these two mutants, and their expression was compared by WB using anti-FLAG.
(C) 293T cells were transfected with pH22 or pH22ΔN that produces WT or ΔN NL4–3 viruses in the presence of indicating amounts of pCMV6 vectors expressing indicated Ser proteins. Cellular Ser protein expression was determined by anti-FLAG and cellular HIV-1 protein expression was determined by indicated antibodies via WB.
(D) Viruses were collected from culture supernatants in (C), and viral infectivity was determined in TZM-bI cells. Infectivity is shown as relative values, with the infectivity of WT viruses produced in the presence of a control vector set as 100%. Error bars represent SDs from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ns, not significant (p > 0.05).
(E) 293T cells were cultured in 10-cm plates and transfected with 4.5 μg pH22ΔN and 4.5 μg pCMV6 vectors expressing indicated proteins. Virions were purified from culture supernatants via ultra-centrifugation and their expression in cells and virions was determined by WB.