Figure 1.
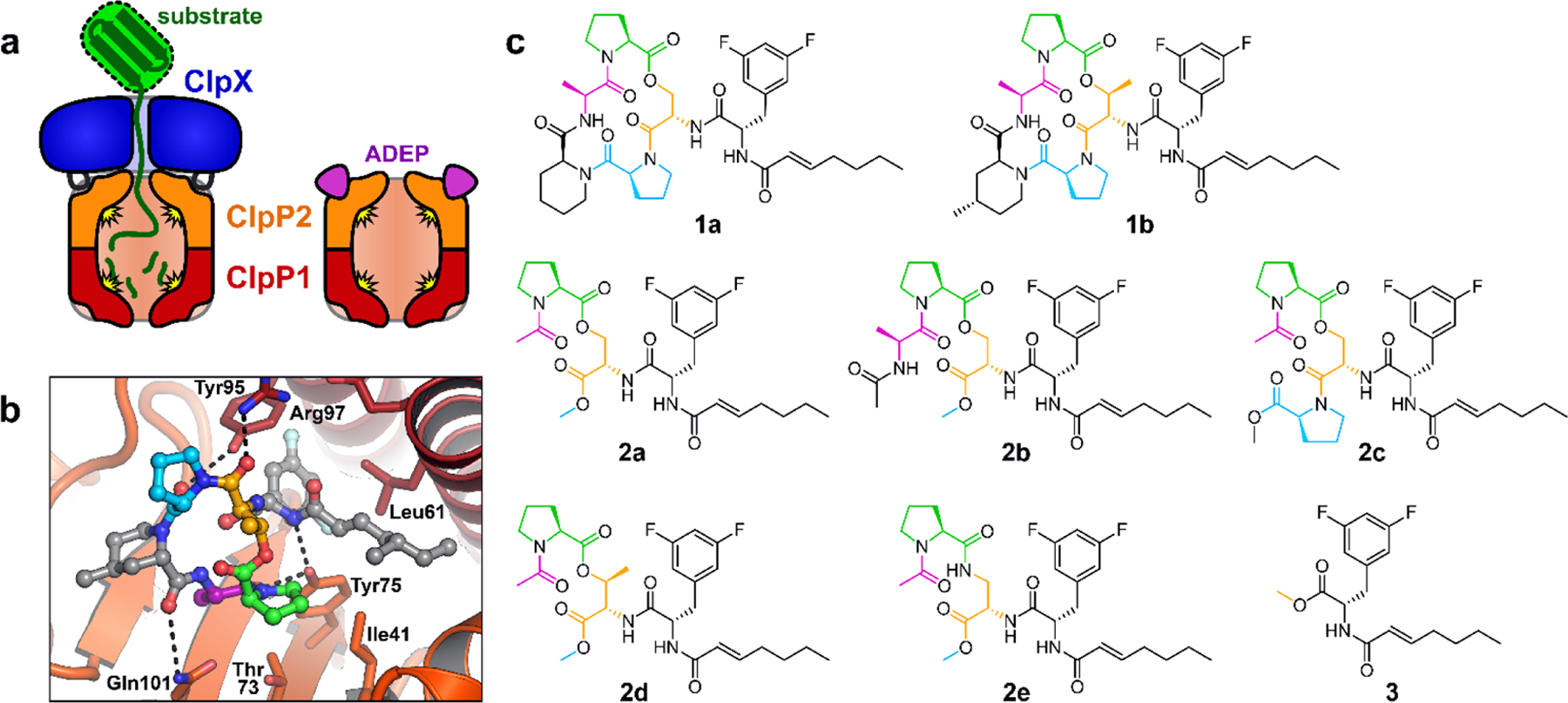
ADEPs bind to the ClpP2 LGF-pockets. (a) Mtb Clp peptidases consist of heptameric rings of ClpP1 and ClpP2 that interact to form a ClpP1P2 heteromer, together with a ring hexamer of ClpX or ClpC1 (not shown) that binds to the surface of ClpP2 via flexible LGF-loops. ADEPs bind to the LGF-pockets of ClpP2, compete with ClpX/ClpC1 binding, and stabilize peptidolytically active ClpP1P2 in the absence of a partner ATPase. (b) ADEP-2B-5Me (ball-and-sticks) is shown bound to a ClpP2 LGF-pocket (ClpP2 subunits shown as dark red and orange cartoons; key residues shown as sticks) from crystal structure 4U0G. The difluorophenylalanine side chain projects into the pocket and the N-acyl chain extends to the right (gray carbons). The macrocycle rests on the surface of ClpP2, to the left. Macrocycle residues shown with colored carbons are present on some of the ADEP fragments described in this study. (c) Structures of ADEPs (1a & 1b), ADEP fragments bearing portions of the macrocycle (2a – 2e), and the N-heptenoyl-difluorophenylalanine methyl ester (3) are colored as in b.
