Figure 7.
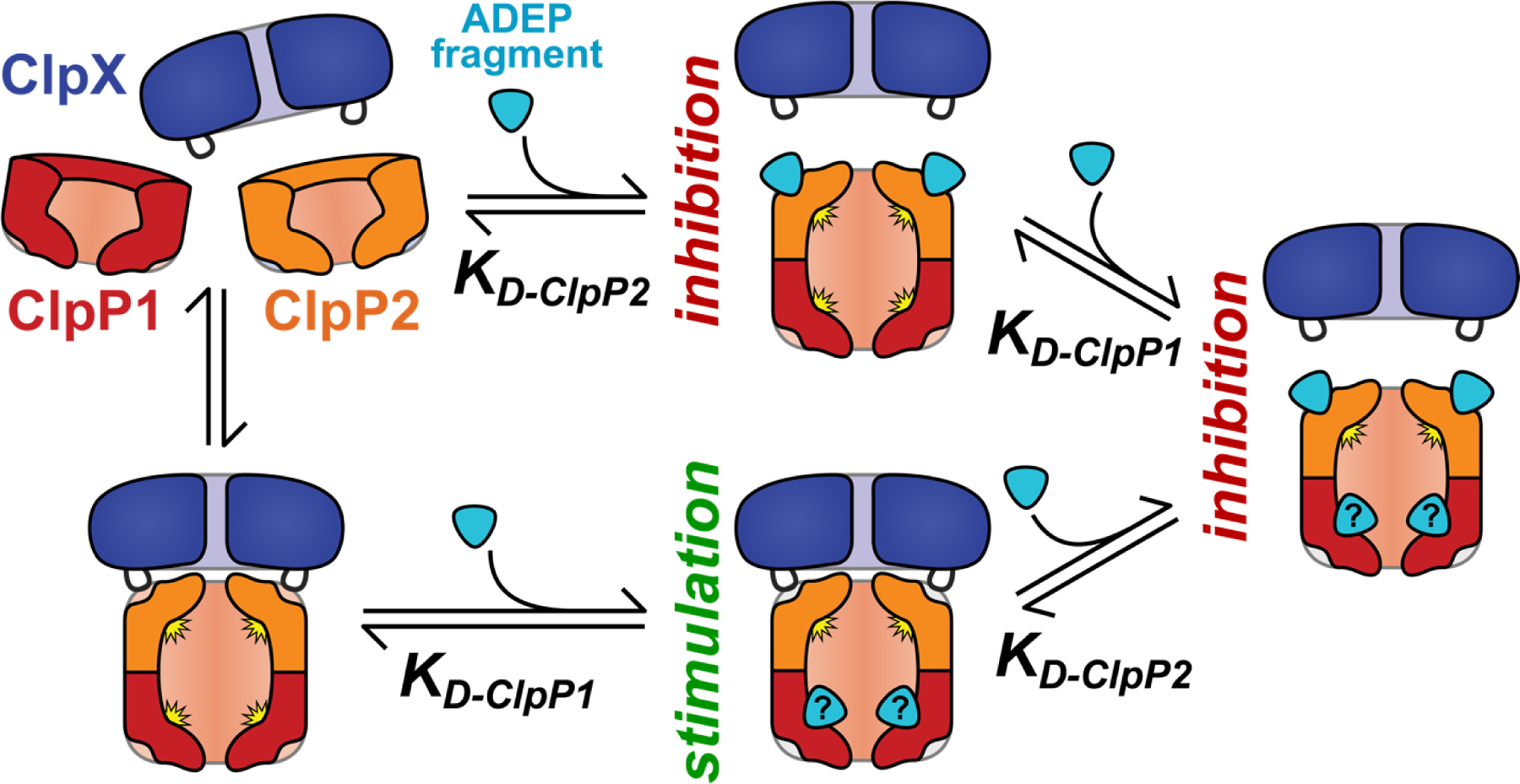
A simple model of the effects of ADEP fragments on ClpXP1P2 activity. Binding of fragment to ClpP2 LGF-pockets leads to competition for ClpX binding and proteolysis inhibition, while fragment binding to a second set of sites stabilizes the active protease. (The second site is illustrated as the ClpP1 active site, but could be elsewhere on the complex.) The net effect of ADEP fragment on proteolysis depends on the relative affinities for the two classes of binding sites. Fragments with KD-ClpP2 << KD-ClpP1 are always inhibitory.
