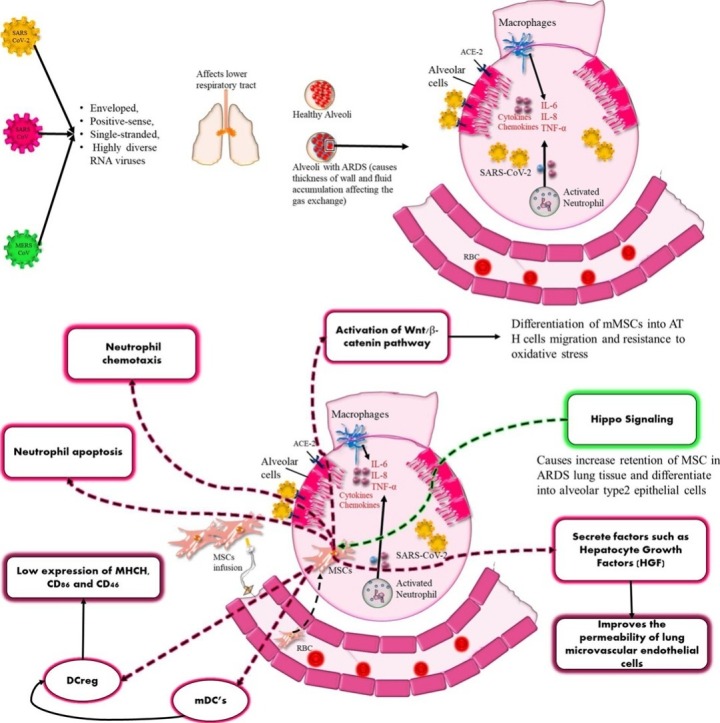
Graphical abstract
Keywords: SARS-CoV-2, Stem cell therapy, ARDS, Immunomodulatory, Coronavirus
Abstract
Background
SARS-CoV-2, which majorly affects the lungs and respiratory tract is thought due to dysregulation of the immune system which causes an immense imbalance of the cytokines. However, till now no standard treatment has been developed in treating the disease. On the other hand, it becomes important to prevent the acute respiratory tract infection due to COVID-19 which is the most dangerous phase leading to increased mortality. Hence this systematic review has been framed by pooling the available data of the use of stem cells in SARS-CoV-2, SARS-CoV, MERS-CoV and ARDS.
Methods
6 literature databases (PubMed, EMBASE, Scopus, Google Scholar, Clinicaltrials.gov, and Clinical trial registry of India) were searched for relevant studies till 10th August 2020 using keywords stem cells, mesenchymal stem cells, cell therapy, SARS CoV-2, SARS Coronavirus, Coronavirus 2, COVID-19, nCoV-19, Novel Coronavirus, MERS CoV, ARDS, acute respiratory distress syndrome.
Results
The observations of this systematic review suggest capability of MSCs in reducing the systemic inflammation and protecting against SARS-CoV-2 as evidenced by the available clinical data.
Conclusion
MSCs can overcome the clinical challenges currently faced by SARS-CoV-2 infected patients, specifically who are seriously ill and not responding to conventional therapies. Though the available clinical data is motivating, still predicting the therapeutic potential of MSCs will be too early in COVID-19. Hence, further studies in a larger cohort of patients becomes a prerequisite to validate their potential efficacy.
1. Introduction
So far, there are seven types of human coronaviruses which have been documented and it includes beta-genera CoV’s and α-genera CoV’s. SARS-CoV-2, SARS-CoV and MERS CoV belong to the family of beta coronaviruses and are enveloped, positive-sense, single-stranded, and highly diverse RNA viruses [1]. These 3 viruses unlike other hCoV’s make the lower respiratory tract more prone towards infection which results in acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) [2].
Regarding genomic homology, SARS-CoV-2 genome was 79.5 % homologous to SARS-CoV, but shared only 50 % homology with MERS-CoV [3]. Again, bat SARS-CoV genome was 87–92 % identical to human SARS-CoV [3]
Structurally also different coronaviruses (SARS-CoV-2, SAS-CoV and MERS-CoV) are similar with spike, M and N proteins being major membrane proteins, among which the spike protein plays a major role in the establishment of a host infection [4]. In all these three coronaviruses, the spike protein consists of two subunits (S1 and S2), out of which receptor binding domain (RBD) of S1 subunit which is mainly responsible for binding of the virus to host receptors and S2 causes the fusion of the virus with the host membrane [5].
Similarly the entry mechanism of the viruses are similar i.e mediated by binding of the RBD to functional receptors on host cell surface where angiotensin converting enzyme (ACE2) is the primary and dominant host receptor but having higher affinity to SARS-CoV-2 than to SARS-CoV [6]. Again high homology was seen between different structural and non-structural proteins (nucleocapsid also between SARS-CoV and SARS-CoV-2) [7]
Again, from pathophysiologic point of view, all the three viruses are similar to a greater extent with occurrence of cytokine storm and ARDS being the major pathological hallmark in all the cases. However, mortality rate is somewhat higher in case MERS-CoV (38 %) and SARS-CoV (9.6 %) [8] compared to SARS-CoV-2 (2.1 %) [9]
Treatment of COVID-19 depends on the clinical condition of the patient such as; patients with mild disease can be treated for their illness at home and not requiring hospitalization. In case of severe or critically ill patients’ hospitalization is required with treatment relating to the condition of the patient. The commonly used therapeutic options for the treatment of COVID-19 ranges from hydroxychloroquine, azithromycin, remdesivir, favipiravir, to immunomodulators e.g. steroid and IL-6 antagonists (tocilizumab, itolizumab), convalescent plasma [10]. Although, these agents showed promising results in in-vitro studies, however, clinical results are not robust. Hence, a lot of other attractive options are being evaluated either as drug therapy or as vaccine e.g. SARS-CoV-2 entry inhibitors, Fusion inhibitors, RdRp inhibitors, NSP-15 inhibitors etc [11]. In few refractory cases even lung transplantation is tried and found to be useful (very limited data) [12,13,11].
In this context, considering the similarity at the level of structure and disease pathophysiology, in this current study, we evaluated the role of stem cells in context to three coronavisuses, which can cause serious human disease (SARS-CoV-2, SARS-CoV and MERS-CoV) and their final outcome (ARDS).
Stem cells are known as unspecialized cells having capacity to differentiate in to cells of whole organism (totipotent) or all the three germ layers except the extra-embryonic structures (pluripotent) or specialized into discrete cells of specific cell lineages (multipotent) or differentiating into only one cell type (unipotent) [14]. Studies have suggested the modulating properties of MSCs where they can modify the adaptive and innate immune response with the suppression of T-cells, maturation of dendritic cells, reduction of B-cell activation and proliferation, inhibiting the proliferation and cytotoxicity of NK cells while promoting the generation of regulatory T-cells with the help of soluble factors (paracrine) or juxtacrine mechanism [15]. Stem cell therapy has already shown promise in other viral infections e.g. Human immunodeficiency virus (HIV) [16], hepatitis B virus [17], virus associated ARDS [18], influenza and other coronavirus associated lung injury [19]. Many studies have indicated the role of up-regulation of interferon stimulating genes (ISG) and interferon-induced transmembrane (IFITM) family of proteins in the protective efficacy of interferons against viral disease [20].
These protective mechanisms provide stem cells the properties of rejuvenation and regeneration which makes them capable of reducing the inflammation, decrease the cell death, secretion of cell protective substances, anti-oxidative effects and improving overall immune function. Further, direct evidence suggest the protection against influenza virus (A/H5N1) by the reversal of lung injury [21].
The immune modulating action of stem cells is mediated by TLRs (TLR, mainly TLR3 and TLR4) present on the surface of MSCs. RNA viruses [act as Pathogen-associated molecular pattern (PAMP)] activate these TLRs, which leads to secretion of certain chemokines like MIP-1α and MIP-1β, RANTES, CXCL9, CXCL10, and CXCL11 etc, which leads to an anti-inflammatory response [22]. These kind of responses can be useful in COVID-19 patients as a similar situtaion of hyperimmune response known as “cytokine storm” has been observed. There has been hypersecretion of pro-inflammatory cytokines which includes IL-2, IL-6, IL-7, G-CSF, IP10, MCP1, MIP1A and TNFα which influences MSCs to release anti-inflammatory molecules (IL-10) with the release of soluble factors like transforming growth factor-β1 (TGF-β1) prostaglandin E2 (PGE2), hepatocyte growth factor (HGF), indoleamine-pyrrole 2,3- dioxygenase (IDO), and nitric oxide (NO) [23]. These in turn decrease the proliferation of activated T-cells and NO on the other hand causes cell cycle arrest by repressing the phosphorylation of signal transducers and transcription of STAT-5 in T-cells. Further, IDO secretion by MSCs leads to apoptosis of activated T-cells and converts tryptophan into kynurenine causing suppression of proliferation of effector T-cells [24].
Besides stem cell therapy, the use of exosomes as a cell free approach has been studied as they possess hypoimmunogenic properties and are enclosed in a lipid bilayer. These properties makes the exosomes extremely stable and suitable for them to migrate to the target organ of damage rather accumulating via blood flow. The combinatorial strategy of antiviral drugs along with immunomodulatory, tissue protective and healing potential of stem cells and their exosomes may reduce the severity of the COVID-19 [25].
Although many studies [26,27] have evaluated the effect of stem cell therapy in COVID-19, however, till now no systematic review have addressed this issue. In this regard, we have pooled the evidence of safety and efficacy of stem cell therapy from SARS-CoV-2, SAR-CoV, MERS-CoV and ARDS data.
2. Material and methods
2.1. Methods
A systematic search was conducted in 6 literature databases (PubMed, EMBASE, Scopus, Google Scholar, Clinicaltrials.gov, and Clinical trial registry of India) for relevant studies till 10th August, 2020using keywords stem cells, mesenchymal stem cells, cell therapy, SARSCoV-2, SARS Coronavirus, Coronavirus 2, COVID-19, nCoV-19, Novel Coronavirus, MERS CoV, ARDS, acute respiratory distress syndrome.
2.2. Inclusion criteria
We included studies (in-vitro and pre-clinical in-vivo and clinical interventional studies) where stem cell or exosomes were used as therapy to modify the outcome of theree human coronavirus that can cause serious human disease (SARS-CoV-2, SARS-CoV and MERS-CoV) and their final outcome (ARDS).
2.3. Exclusion criteria
We excluded studies involving other corona-viruses which do not cause serious human disease (229E, NL63, OC43, and HKU1), in-silico studies, and studies including intervention other than stem cell/cardiosperes/exosomes.
2.4. Extraction of data and analysis
Title and abstract screening were conducted independently by Saniya Mahendiratta, Seema Bansal, Phulen Sarma and Harish Kumar using pre-defined exclusion and inclusion criteria. Ajay Prakash and Bikash Medhi were consulted for resolving the differences in opinion. Data extraction and subsequent full-text review was done by Saniya Mahendiratta and Seema Bansal using Cochrane data extraction form. Cross checking of the data retrieved for each article was done by other authors.
3. Results
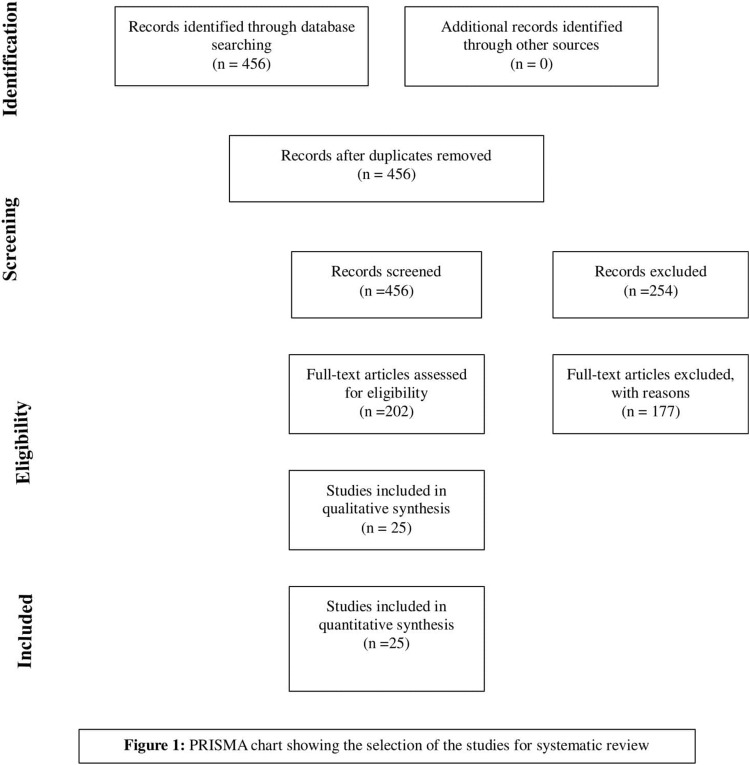
The systematic search of the databases yielded 456 studies. With the removal of zero duplicate 456 articles were reviewed by abstract and title. With initial screening only, 202 met the inclusion criteria and underwent for full text evaluation. Of these, studies 177 studies were not fulfilling our criteria were removed, and final list include 25 articles (Prisma Chart Fig. 1 ).
Fig. 1.
Prisma Flow Chart.
3.1. In-vitro studies
3.1.1. In-vitro studies for SARS-CoV-2, SARS-CoV and MERS-CoV
After deliberate search of 4 databases with appropriate keywords, we couldn’t find any relevant data regarding in-vitro evaluation of safety or efficacy of stem cell in COVID-19 or SARS-CoV-2, SARS-CoV and MERS-CoV.
3.1.2. In-vitro studies for Acute respiratory distress syndrome (ARDS)
(Table 1 )
Table 1.
In-vitro stem cell therapy in Acute Respiratory Distress Syndrome (ARDS).
| Author, Year | Aim of the study | Condition | Intervention/ Details of stem cell use | Outcome |
|---|---|---|---|---|
| Pati et al. 2011 [29] | To study the effects of conditioned media (CM) from MSCs and MSC-PEC co-cultures on Pulmonary endothelial cells permeability. | ALI/ARDS due to Hemorrhagic Shock (HS) | BMMSCs | Inhibition of permeability of PEC. Restoration of VE-Cadherin and B-catenin (chief regulators of vascular integrity and permeability). MSC-PECCM inhibited TNF-α mediated increase in ICAM-1, and VCAM-1 (suggestive of contact-mediated effect) |
| Chen et al. 2015 [28] | To investigate the impact of human umbilical cord Wharton’s jelly-derived MSC (hUC-MSC) secreted factors on alveolar epithelial cells under septic conditions |
Liposaccharide induced ARDS | hUC-MSC | MSC-CM under septic conditions promoted proliferation in A549 cells under septic conditions. Also caused wound healing and a significant increase in A549 cells |
| Masterson et al. 2018 [30] | To evaluate the effects of syndecan 2 (CD362)–expressing human mesenchymal stromal cell enhance resolution after ventilator induced lung injury |
hMSCs | Conditioned medium from CD362+ human mesenchymal stromal cell attenuated interleukin 1β–induced nuclear factor-κβ activation in type II alveolar A549 cells. Also rate of wound closure was increased in A549 alveolar epithelial cultures after 24 h of wound injury. | |
| Xiang et al. 2017 [31] | Evaluated the effects of MSC in attenuating the lung inflammation with repairing effecton damaged lung. | LPS-induced lung injury | Menstrual blood-derived stem cells (MenSCs) | Lung injury was attenuated, reduction of inflammation exhibited in the form of decreased levels cytokines. Increased proliferation and decreased apoptosis shown by PCNA (increased expression) and caspase-3 (decreased expression). Also, there was increased protein levels VE-cadherin, β-catenin and PI-3 K and decreased levels p-gsk3β, p-src and p-β-catenin. |
| Yi Fong Su et al [33] | Studied the effects of iPSC on the migration of human neutrophils isolated from ARDS patients | LPS-induced lung injury | Induced pluripotent stem cells (iPSC) | Significant decrease in neutrophil migration |
| Yi Fong Su et al [32] | Evaluated the effects of MSC-CM on the apoptosis of pulmonary neutrophils. | LPS-induced lung injury | MSC-CM | Higher apoptosis was observed when treated with MSC-CM compared to LPS alone |
3.1.2.1. Effect of human umbilical cord Wharton’s jelly-derived MSC’s (hUC-MSC) secreted factors in Lipopolysaccharide (LPS) induced lung injury
Chen et al. [28] in their study investigated the effects of MSC-conditioned medium (CM) on two cell lines: primary human small airway epithelial cells (SAEC) and human alveolar epithelial A549 cells (AEC). Wound healing assay, modified MTT assay, and trans-well migration assay was performed to evaluate MSC-CM effect on proliferation and migration of SAEC and AEC. The stem cell-CM treated AEC and SAEC cells showed evidence of proliferation and effective wound healing compared to control or LPS group. On further exploring the molecular pathways, they found the involvement of JNK, P38 and ERK MAPKin the protective effect of MSC-CM on cellular proliferation, wound healing and migration in AEC and SAEC.
3.1.2.2. Effect of Pulmonary epithelial cells and MSC-PEC conditioning medium on PEC permeability
Pati et al. [29] evaluated the effects of Conditioned Media (CM) isolated from Mesenchymal stem cells (MSC) and MSC-PEC-co-cultures on pulmonary epithelial cells (PEC). They observed their inhibitory activity on the permeability of PEC which was due to the preserved adherens junctions (β-catenin and VE-cadherin). Further, MSC-PEC co-cultures also inhibited adhesion molecule (VCAM-1 and ICAM-1) and leukocyte adhesion expression. This study actually demonstrated the therapeutic potential of MSC in acute lung injury (ALI) caused by Hemorrhagic shock (HS).
3.1.2.3. Effects of CD362+ human mesenchymal stromal cells in modulating the pulmonary functions
Masterson et al. [30] determined the potential of conditioned medium from CD362+ human mesenchymal stromal cells in type II alveolar A549 cells. They performed NF-κB activation assay, wound healing assay and macrophage phagocytosis assay. It was observed that conditioned medium attenuated interleukin-1β induced NF-κB activation and also the rate of wound closure. It was also shown that these effects were significantly more effective compared to heterogeneous human mesenchymal stromal cells.
3.1.2.4. Effects of Menstrual blood-derived stem cells (MenSCs) in LPS induced lung injury
Xiang et al. [31] performed transwell experiments to show the migration ability of MenSCs. Observations showed 17.8 % migration of MenSCs into the lower layer of the membrane from the transwell inserts in LPS group compared to the control group which showed migration in only 9.5 % of cells. For evaluating the effectiveness of MenSCs on apoptosis and cell viability in BEAS-2B cells (human lung epithelial cells), LPS exposure was given to these cells which showed significant reduction in viability and higher rate of apoptosis. This was reversed when treated with MenSCs with reduction in apoptotic rate compared to normal BEAS-2B cells (control).
3.1.2.5. Effects of induced pluripotent stem cells (iPSC) on human neutrophil migration
Yi Fong SU et al. [32] studied the migration of human neutrophils isolated from ARDS patients by treating them with iPSC using transwell migration model. A significant decrease was seen in neutrophil migration induced by LPS when treated with iPSC. Similarly, in their another study [33]. MSC-CM effect on apoptosis of pulmonary neutrophils was studied. These neutrophils were isolated from bronchoalveolar lavage fluid (BALF) samples from mice and cultured with or without LPS and MSC-CM treatment. Higher apoptosis was observed when treated with MSC-CM in comparison to LPS alone.
3.2. In-vivo studies
(Table 2 )
Table 2.
In-vivo (Preclinical) stem cell therapy in SARS-CoV-2 and Acute Respiratory Distress Syndrome (ARDS).
| Author, Year | Aim of the study | Condition | Intervention/ Details of stem cell use | Number of Animals used | Outcome |
|---|---|---|---|---|---|
| Liu et al [34] | Vaccine based platform by SARS-CoV-2 proteins expressing MSCs | COVID-19 | MSC-SARS-CoV-2-N cells | 8 C57BL6 mice | Antibody production was seen in almost all the immunized mice (7/8) after 20 days. Body weight remained same or increased with no severe symptoms. Responses were for Nucleocapsid (N) protein even after transfection with Spike and Membrane proteins of SARS-CoV-2. Limitation:This approach of cell therapy as a vaccine can have one potential risk of uncontrolled proliferation and malignancy if residual cells remains in the body. |
| Devaney et al. 2015 [39] | Determination of efficacy and mode of action of human MSCs (hMSCs) in acute lung injury | E. coli induced lung injury (ARDS) | Human Mesenchymal stem cells (hMSC’s) | 48 Sprague Dawley rats | Reduction of E. coli pneumonia, lung injury, bacterial loads in lungs due to suppressed inflammation and improved survival. The effectiveness of intratracheal hMSC administration was equal to intravenous hMSC. Cryopreserved hMSCs were also effective with less efficacy of hMSC secretome. Increased the alveolar concentrations of LL-37 |
| Shalaby et al. 2014 [40] | To assess the effects of MSC in the attenuation of ALI by controlling oxidative stress | E. coli induced lung injury | BMMSCs | Eighty male Balb c mice, in groups of 4 with 20 animals in each group. | Reduced survival rate improved in both pre and post ALI, with higher survival rate in pre-ALI group compared to post ALI. (pre = 19/20 and post = 17/20). Reduced the pulmonary edema, significant reduction of MPO activity and significant rise of anti-oxidant enzyme activities, GSH and TAC levels with reduced MDA levels. |
| Masterson et al. 2018 [30] | To evaluate the effects of syndecan 2 (CD362)–expressing human mesenchymal stromal cell enhance resolution after lung injury caused by ventilator. |
E. coli and ventilator induced lung injury | Human Mesenchymal stem cells (hMSCs) | 40 Adult male Sprague Dawley rats (divided into 4 groups and 10 animals in each) |
Attenuation of injury by E. coli, improved arterial oxygenation and lung compliance, reduction in bacterial load and decreased structural injury. Also enhanced the resolution after ventilator induced injury with the restoration of arterial oxygenation and lung compliance, resolving lung inflammation and restoring the histologic structure. |
| Xiang et al. 2017 [31] | Evaluating the effects of MSC in attenuating the lung inflammation and repairing action in damaged lung. | LPS-induced lung injury | MenSCs | Lung injury was attenuated, reduction of inflammation exhibited in the form decreased levels of IL-1β and increased IL-10. Increased proliferation and decreased apoptosis shown by PCNA (increased expression) and caspase-3 (decreased expression). Also, there was increased protein levels of VE-cadherin, β-catenin and PI-3 K and decreased levels p-gsk3β, p-src and p-β-catenin. | |
| Chen et al. 2018 [36] | To explore the effects of Heme oxygenase (HO‐1‐) modified bone‐marrow–derived MSCs (MSCs‐HO‐1 |
LPS-induced lung injury | Bone‐marrow–derived MSCs | 8‐week‐old Wistar rats (divided into 4 groups: NS, lenti‐HO‐1‐GFP, MSC, and MSC‐HO‐1) |
Survival rate was significantly increased in all the groups compared to NS, and further improved in MSC‐HO‐1 group. Lung injury and its score was reduced, with decreased counts of neutrophil in BALF and retardation of lung water content. Reduction in TNF-α and IL-1β levels. |
| Zhang et al. 2013 [38] | To study the therapeutic effects ASC-based therapy | LPS-induced lung injury | (hASCs) and (mASCs) Adipose-derived stem cells | C57Bl/6 mice | Had anti-inflammatory effects, leukocyte migration into the alveoli was decreased (for example neutrophil). The expression of proinflammatory cytokines suppressed and enhanced anti-inflammatory cytokine (IL-10) levels. |
| Zhao et al. 2008 [41] | To determine the effect of MSC engraftment in the protection of lungs | Bleomycin induced lung injury | BMMSCs | Adult Sprague-Dawley rats | Alveolar wall thickness, fibroblast number and collagen quantity in the lung interstitium were significantly reduced, most alveoli were intact. Laminin (LN), hyaluronan (HA) and Hydroxyproline content was decreased indicating amelioration of injury in the lung and fibrosis. |
| Kumamoto et al. 2009 [43] | To test the engraftment of minimally cultured BMMSCs on amelioration of progressive fibrotic lung injury. |
Bleomycin induced ling injury | 2-h adherent BMMSCs or 9-day cultured BMMSCs |
C57BL/6 J mice (6–8 weeks old) | Weight was restored and animals survived (80 % survival in (2-h adherent BMMSCs and 74 % in 9-day cultured BMMSCs). Attenuation if bronchoalveolar lavage (BAL) and neutrophil count. Downregulation of type 1 pro-collagen indicating reduction of inflammation and lung fibrosis. |
| Wakayama et al. 2015 [42] | To evaluate the use of stem cells derived from human exfoliated deciduous teeth (SHEDs) or SHED-derived serum-free conditioned medium (SHEDCM) | Bleomycin induced lung injury | human exfoliated deciduous teeth (SHEDs) | Adult 7- to 9-week-old C57BL/6 J mice | Improvement in weight loss and survival rate, reduced inflammatory cells and fibrosis. There was suppression of pro-inflammatory cytokines ((IL-6, IL-1β,TNF-α) with the induction of anti-inflammatory M2-like lung macrophages |
3.2.1. In-vivo studies for SARS-CoV-2
A study by Liu et al. [34] demonstrated a novel vaccine platform for treating COVID-19 by SARS-CoV-2 proteins expressing MSCs. They administered MSC-SARS-CoV-2-N cells in C57BL6 mice and observed antibody production in almost all the immunized mice (7/8) after 20 days in which 50 % had strong positive antibody expression. The body weight of the mice remained same or increased with no severe symptoms after the MSC administration. It was also seen that responses were again for Nucleocapsid (N) protein even after transfection with Spike and Membrane proteins of SARS-CoV-2. Further, they performed PCR for N protein in the blood and tissue which demonstrated clearing of MSCs by the immune system. This approach of cell therapy as a vaccine can have one potential risk of uncontrolled proliferation and malignancy if residual cells remains in the body.
3.2.2. In-vivo studies for SARS-CoV and MERS-CoV
After deliberate search of 4 databases with appropriate keywords, we couldn’t find any relevant data regarding in-vivo evaluation of safety or efficacy of stem cell in COVID-SARS-CoV and MERS-CoV.
3.2.3. In-vivo studies for ARDS
3.2.3.1. LPS induced acute lung injury
3.2.3.1.1. MSC
Hu et al. [35] studied the effectiveness of MSC in LPS induced lung injury in Sprague Dawley rats. They measured vascular permeability in lungs, performed histopathology and also checked the cytokine levels. Further, they also determined whether expression of hepatocyte growth factor (HGF) in MSC will improve the vascular permeability and lung injury caused by LPS. MSC administration improved the vascular permeability in the lungs with an increase in lung score in histopathological findings specifically with HGF. Additionally, there was a decrease in cytokine levels and rate of apoptosis with an increased expression of VE-cadherin in MSC group expressing HGF compared to acute lung injury (ALI) group. Xiang et al. [31] in his study observed amelioration of lung injury induced by LPS with the administration of MenSCs in C57 mice. They assessed migration, inflammatory responses, proliferation and apoptosis. It was observed that MenSCs were able to migrate and get retained in the injured lungs, attenuating the inflammation with improvement in histopathology. Further, reduced proliferating cell nuclear antigen (PCNA) expression and increased caspase-3 expression was observed indicating proliferative and anti-apoptotic actions of MenSCs compared to LPS group. Chen et al. [36] investigated Heme oxygenase-1 modified MSC (MSC-HO-1) protective effects in LPS induced acute lung injury in Wistar rats. Survival rate and score of lung injury was significantly improved with the administration of MSC-HO-1. There was a reduction in inflammation exhibited by an enhanced production of keratinocyte growth factor, HGF and IL-10 in MSC-HO-1 group compared to LPS group. In a study by Li et al. [37] repairing capacity of MSCs were assessed with downregulated Hippo-signaling pathway in LPS induced lung injury in C57BL/6 mice. Significant retention of MSCs were seen in lungs, with decreased lung edema and improved lung permeability. There was also decreased inflammation, improved pathological changes with reduced pulmonary fibrosis.
3.2.3.1.2. Adipose derived stem cell
Zhang et al. [38] compared the therapeutic effects of Human adipose derived stem cells (hASCs) and mouse derived stem cells (mASCs) in C57Bl/6 mice. It was observed that there was an increase in the body weight of the animals in both the groups compared to LPS group but significance in improvement was seen with mASCs. To determine the effects on the integrity of the pulmonary vascular leakage and alveolar-capillary membrane barrier, protein and albumin levels were assessed in bronchioalveolar lavage fluid (BALF) which decreased with the treatment of hASCs and mASCs with much better effect with mASC. Further,there was decreased inflammation and leukocyte migration into the alveoli but mASCs was more potent compared to hASCs in terms of therapeutic effects.
3.2.3.1.3. Endotoxin induced lung injury:MSC’s
Su et al. [32,33] in his studies assessed the neutrophil apoptosis with the administration of MSC-CMin wild-typeC57BL/6 male mice and also evaluated the activity of induced pluripotent stem cells (iPSCs) in lessening the chemotaxis of neutrophils. In the former study the apoptosis in the BALF neutrophils was enhanced with the reduction in expression of Bcl-xL and Mcl-1. While in the latter study chemotactic responses towards endotoxin were with the reduction of chemokine (C-X-C motif) receptor 2 (CXCR2) expression in mouse peripheral blood neutrophils. Additionally, it was investigated that the effects of the latter study were due to reduced CXCR2 expression and augmented GRK2 activity.
3.2.3.1.4. E. coli induced lung injury: MSC’s
Lung injury can also be caused by prolonged pneumonia due to exposure to E. coli, which can be treated by MSCs. This has been shown in one of the studies by Devaney et al. [39] where he compared the efficacy of intrathecal and intravenous administration of MSCs along with cryopreserved MSCs and its secretome in Sprague Dawley rats. Results showed reduced severity of pneumonia with improved survival and decreased lung injury. The bacterial load was also decreased with a reduction in inflammation. It was also observed that intrathecal administration was much more effective with higher efficacy of cryopreserved MSC than the secretome. Shalaby et al. [40] in his study demonstrated the anti-oxidant effects of systemic injection of MSC pre and post injury in Balb c mice. It was observed that pulmonary edema was reduced and lung injury was attenuated. Also, anti-oxidant enzyme activities were increased with a reduction of malondialdehyde and myeloperoxidase activity. It was also assessed that the effects were profound when MSC was given before injury compared to post-injection. Masterson et al. evaluated the effects of CD362+ (syndecan-2-positive) human mesenchymal stromal cells in E. coli induced lung injury in Sprague Dawley rats. Results showed improvement in arterial oxygenation, lung compliance, also reduction in bacterial load and structural injury in MSC group compared to vehicle.
3.2.3.1.5. Bleomycin induced lung injury: MSC
Zhao et al. [41] illustrated therapeutic efficacy of MSC engraftment in lung injury caused by Bleomycin (5 mg/kg) in rats. It was observed that MSC attenuated the lung injury exhibited in the form of decreased hydroxyproline in lungs, laminin and hyaluronan in bronchoalveolar lavage fluid (BALF), which are the markers of fibrosis and lung injury. Even cytokines expression which includesTGF-β1, PDGF-A, PDGF-B, and IGF-I were reduced significantly thus documenting the favorable effects of MSC engraftment. Wakayama et al. [42] in his study showed amelioration of the lung injury and weight loss by treating the mice with stem cells derived from human exfoliated deciduous teeth (SHEDs) or SHED-derived serum-free conditioned medium (SHEDCM). Even the recovery levels were increased and inflammatory responses were decreased in the treatment group. Kumamoto et al. [43] assessed the effects of minimally cultured BMMSCs in female C57BL/6 J mice. He observed restoration of the weight loss and increased survival compared to control rats (weight loss recovery of 3/10). Further, anti-fibrotic action was also shown with the downregulation of type I procollagen transcription.
3.3. Clinical studies
(Table 3 )
Table 3.
Clinical studies of Stem cell therapy in patients with SARS-CoV-2 and ARDS.
| Author Year | Study design | Status | Condition | Population | Intervention/ Details of stem cell use (Stem size) | Control (sample size) |
Outcome | Remark |
|---|---|---|---|---|---|---|---|---|
| Leng et al, 2020 [27] | Pilot study | Completed | COVID-19 | 18−95 years Total: 7 (1 critically severe, 4 severe, 2 common type) |
Mesenchymal Stem cell (MSC) | 3 severe type | Primary Outcome: All symptoms (high fever, weakness, breathlessness and low oxygen saturation) disappeared in all the patients 2−4 days after the treatment with no adverse effects. RT-PCR turned negative for nCOV-19 Efficacy outcome: For critically severe patient (decrease in CRP, increase in oxygen saturation, respiratory rate decreased to normal range, Chest CT: showed ground-glass opacity and decreased pneumonia infiltration. |
Patient enrolled in this study were COVID-19 positive who had no improvement with standard treatment. Had no history of tumors. |
| Zhang et al, 2020 [44] | Case report | Completed | COVID-19 | Critically ill 54-year old male patient having cough, fever and tightness of chest from 4 days | Umbilical cord Wharton’s jelly-derived MSCs (hWJCs) | NA | Efficacy Outcome: Recovery of Shortness of breath, decrease in CRP, increased lymphocyte count, decrease in inflammatory mediators and the patient was discharged. No ADR reported and COVID-19 nucleic acid turned negative after 6 days. |
No malignancy And from 3 months the patient should not be included in any other clinical study |
| Alturi et al,2020 [26] | Case report | Completed | COVID-19 | 65y/o with severe pneumonia, respiratory failure and multiorgan failure requiring mechanical ventilation |
doses each of 50 million allogeneic umbilical cord stem cells with conventional therapy |
NA | Vital signs stabilized, not dependent on ventilator. After the infusion patient was negative for the virus on throat swabs after 2 days. |
This was the first coronavirus case treated with umbilical cord cells reported from China. |
| Liang et al,2020 [45] | Case report | Completed | COVID-19 | 65-year-old woman | hUCMSCs administrated intravenously for three times with antibiotics and thymosin α1 | NA | Reduced serum levels of bilirubin, CRP, ALT/AST along with improvement of vital signs. Decreased counts of white blood cells, and neutrophils and increase in lymphocyte count and throat swabs tests reported negative | |
| Peng et al, 2020 [46] | Case report | Completed | COVID-19 | 66-year old female | Convalescent plasma along with UC-MSCs | Absolute lymphocyte count was improved after twice administration of convalescent plasma and no infusion or allergic reactions were seen after UC-MSC administration. | ||
| Singh et al,2020 [47] | Case series | Completed | COVID-19 | 6 COVID-19 critically ill patients (19–75 years) | Allogeneic cardiosphere-derived cells (CDCs) (CAP-1002) along with hydroxychloroquine and tocilizumab | Contemporaneous control group (n = 34) | No ADR reported. 4 patients improved clinically with the exception of 2 who remained critically ill but stable. 6 (18 %) deaths in control group |
|
| Sengupta et al,2020 [25] | Prospective nonblinded nonrandomized primary safety trial |
Completed | COVID-19 | 24 COVID-19 patients (18–85 years) | Exosomes derived from allogeneic BMMSCs | 83 % survival rate, 71 % patients recovered, 13 % remained critically ill but stable and 16 % expired unrelated to treatment. No ADR reported. | Exclusion criteria included pre-existing cardiopulmonary, hematologic diseases, liver and kidney dysfunction, metabolic disorder with irreversible coagulopathy, pregnancy, immunodeficiency secondary to other viruses, or disseminated intravascular coagulation. |
|
| Simonson et al [51] | Case reports | Completed | ARDS | 40 and 58years old men | MSC | The improvements in both the patients was broad with a decrease in inflammatory markers. Additionally, level of surfactant protein B was increased in bronchial alveolar fluid (BAL) which is an indicator of recovery of alveolar epithelial function. | ||
| Zheng et al. 2014 [48] | Single-center, randomized, double-blind, placebo-controlled study. |
Completed | ARDS | Total: 12 ARDS: 6 |
Human adipose derived MSC’s | Placebo: 6 | Study drug was well tolerated with 1 patient with diarrhea and other with rash in MSC group (resolved within 48 and 24 h) 1: Death in MSC arm and 2 in control arm (not related to study drug) Improvement in oxygenation index. Significant lowering of SP-D levels at day 5 compared to day 0. |
Limitations: Small sample size |
| Wilson et al. 2015 [49] | Multicentre, open-label, dose-escalation, phase 1 clinical trial. | Completed | ARDS | Total: 9 Divided into 3 groups with 3 patients in each group (according to doses) |
Allogeneic BMMSCs | Well tolerated 3 patients had serious ADR not related to MSC, out of which 2 patients died. Mean Lung injury score was improved from baseline till day 3 in all 3 groups. Greatest decrease in high dose (high dose 2·9 to 1·6 [–45 %]) and lowest in low dose group 3 to 2·1 [–30 %]) Similar results with SOFA (sequential organ failure assessment) score: (high dose 7 to 3·7 [–48%] and low dose 8 to 7·7 [–4%]) |
||
| Matthay et al. 2018 [50] | Double-blind, Randomized, placebo-controlled, phase 2a trial |
Completed | ARDS | Total: 60 MSC: 40 |
Mesenchymal stem cells | Placebo: 20 | Infusion of 20 h caused fatal cardiopulmonary arrest but death not related to MSC. Mortality at day 28 and 60 was non-significantly higher in MSC group than placebo. Ventilator free and organ failure free days were non-significantly few in MSC group than placebo. Oxygenation index had non-significant reduction in MSC group compared to placebo. |
3.3.1. Clinical studies of SARS-CoV-2
3.3.1.1. Mesenchymal stem cells
One of the pilot trials was conducted by Leng et al. [27] in 7 COVID-19 patients using intravenous Mesenchymal Stem cell (MSC) transplantation to assess its therapeutic potential placing 3 severe patients in placebo group. The assessment was done for 14 days after receiving the MSCs which showed no adverse reactions with impressive efficacy outcomes. This was in terms of reduced levels of C-reactive protein (10 fold), increased oxygen saturation (89 %–98 %) with reduction in fever, shortness of breath and pneumonia infiltration and patients testing negative on 13th day after transplantation. Immune profile improved with decrease in pro-inflammatory cytokines and vice-versa for anti-inflammatory cytokines (IL-10) (p < 0.05) was seen. Study suggested the absence of ACE2 and TMPRSS2 with high expression of certain trophic factors as the possible immunomodulatory mechanism of MSC.
3.3.1.2. Mesenchymal stem cells derived from umbilical cord
Alturi et al. [26] presented the first case of a COVID-19 patient from China aged 65-year old treated with umbilical cord MSC (3 doses each of 50 million 3 days apart allogenic umbilical cord MSC). Similarly Zhang et al. [44] presented the case of 54-year old man intravenously infused with hUC-MSC. In both the cases patients were critical with severe pneumonia, low oxygen saturation, shortness of breath, respiratory failure and multiorgan failure requiring mechanical ventilation and were given simultaneously the standard treatment. Related to above study the efficacy outcome was contributed by immunomodulatory effects with no known adverse or hypersensitivity reactions.
Another study by Liang et al. [45] who presented a case of a SARS-CoV-2 infected critically-ill female aged 65-years. She presented with fatigue and fever and was prescribed antibiotics and phlegm reducing agents as supportive agents. After some time, she complained of chest tightness with glass opacity of lungs on X-Ray and her RT-PCR was covid positive. The patient was treated with anti-viral agents following the diagnosis and treatment guidelines for SARS-CoV-2. The patient condition worsened with acute diarrhea and acute respiratory failure and she was diagnosed as critically ill patient. These results indicated that the current therapy she was receiving was not working properly and hUCMSCs was proposed. After she received the first dose, no adverse effects were observed indicating the tolerability and her vital signs were also improved. After the second administration the T-cell counts normalized and it was observed that initial therapy of α-thymosin when combined with hUCMSCs greatly reduced the inflammation. The patient was relieved of ICU and her vital signs recovered to the normal levels and she tested negative for the virus.
Peng et al. [46] also presented the case of a 66-year old female who had sore throat, cough and fever after coming in contact with COVID-19 patient. She was tested positive for SARS-CoV-2 at day 10 and was considered severe according to guidelines of COVID-19 diagnosis and treatment. Convalescent plasma was given to the patient along with the UC-MSCs and was observed for certain clinical, laboratory, and radiological parameters. It was observed that absolute lymphocyte count was improved after twice administration of convalescent plasma and no infusion or allergic reactions were seen after UC-MSC administration. After some days she tested negative, recovered and discharged from the hospital.
3.3.1.3. Mesenchymal stem cells derived from Bone Marrow (BMMSCs)
In a prospective non-randomized open-label cohort study by Sengupta et al. [25] utilized exosomes derived from allogenic BMMSCs (ExoFlo). The evaluation was in terms of efficacy and safety in 24 confirmed COVID-19 patients treated with an i.v dose of 15 mL. 83 % survival rate was observed with a recovery rate of 71 % (17/24), stability in 13 % (3/24) and mortality unrelated to treatment was 16 % (4/24). Laboratory investigations showed significant reduction in absolute neutrophil count (p-value <0.001) with alleviated levels of acute phase reactants, C-reactive protein, downregulating cytokine storm and restoring immunity again implying a key action on immune functions. Further RCT’s are required to explore its therapeutic potential.
3.3.1.4. Cardiosphere derived cells
Another cell-based therapy (CAP-1002, derived from allogenic cardiospheres) was assessed for its safety and effectiveness by Singh et al. [47]. The assessment was done in 6 critically ill COVID-19 patients (age range of 19–75 years) who were all on ventilatory support with 34 critically ill patients in control group. All patients survived with 4 discharged and 1 still on respiratory support compared to 18 % mortality in control group. Results were well correlated with diminished levels of ferritin and absolute lymphocyte counts, still suggesting the role of cell-based therapies in modifying the immune responses. Cardiosphere derived cells are progenitor cells which have the property of regeneration and hence has been included in this systematic review.
3.3.2. SARS-CoV and MERS-CoV
After deliberate search of 4 databases with appropriate keywords, we couldn’t find any relevant data regarding clinical studies evaluating the safety or efficacy of stem cell in SARS-CoV and MERS-CoV.
3.3.3. Clinical studies of ARDS
3.3.3.1. Allogeneic adipose-derived MSCs
A single-center, double-blind, placebo-controlled, randomized study designed by Zheng et al [48] was done in which adipose-derived MSCs was systemically administered to assess its safety and feasibility in ARDS patients. Twelve patients were recruited with at least 18 years of age as the eligibility criteria and diagnosis was made with a PaO2/FiO2 ratio of < 200 within 48 h. Six patients were allocated in MSC group and other 6 in placebo. Single dose of 1 × 106 cells/kg or saline was administered to the patients as a i.v. infusion over 60 min in both the groups with 28 days as the follow-up period. No ADR was reported with the exception of 2 patients (1 had diarrhea and the other had rash on the chest, which resolved in 48 and 24 h). 1 patient died in MSC arm and 2 in placebo group but the deaths were not related to the treatment. In terms of efficacy, oxygenation index was improved significantly from baseline compared to placebo group with no significant difference in improvement in PaO2/FiO2 between two groups at all the time points. ARDS biomarker SP-D levels were significantly reduced at day 5 compared to day 0 in MSC group, and similar trend was seen with IL-6 levels but the change was not significant.
3.3.3.2. Allogeneic bone marrow-derived MSCs
Wilson et al. [49] performed a multicentre, open-label, dose-escalation, phase 1 clinical trial with 9 ARDS patients to evaluate the safety and tolerability of MSC. Patients were divided into 3 cohorts with 3 in each group based on different doses of MSC. Low dose: (1 million cells/kg), Intermediate dose: (5 million cells/kg) and high dose: (10 million cells/kg PBW). Assessment was based on Lung injury score (LIS) and Sequential Organ Failure assessment (SOFA). Within 60 days of study infusion 2 patients died out of 9 (mortality rate 22 %). It was noticed that the first death was on day 9 in low dose group, and other was on day 31 with intermediate dose. LIS score improved in all the 3 groups with highest improvement in high dose and lowest improvement in lower dose, similar results were seen with SOFA scores. There was no significant decrease biomarker concentration at baseline, after 6 h and on day 1and 3.
Another study by Matthay et al. [50] which was a prospective, randomized trial, multicentre, double-blind was performed to assess the safety of MSC in 60 ARDS patients in which 40 were in MSC group and 20 in placebo arm. There was no MSC related hemodynamic or respiratory adverse events, but there was death of 1 patient within 24 h of infusion which was unrelated to MSC. Mortality at day 28 was 12 (30 %) andat day 60 was 15 (38 %) which was higher non-significantly in MSC group compared to placebo(3 (15 %) at day 28 and 5 (25 %) at day 60) with OR of 2.4 (0·5–15·1) (at day 28) and 1.8 (0·5–7·6)(at day 60) but there was a reduction in the oxygenation index which was not significant compared to placebo.
Simonson et al. [51] presented two cases, first of an old man aged 58 years who had hospitalization after the start of cough, high fever and dyspnea. He had history of hypertension and was diagnosed with influenza A H1N1. The other patient aged 40 year man, with no past medical history and was hospitalized because of loss in weight,malaise, gingival hyperplasia, fever and night sweats. Acute Myeloid Leukemia (AML) was diagnosed for which chemotherapy was started. The condition of the patient deteriorated progressively where ARDS was a possible contributing factor according to Berlin criteria. The condition of both the patients worsened even after the conventional and supportive therapy and hence was administered MSCs. The improvements in both the patients was broad with a decrease in inflammatory markers. Additionally, level of surfactant protein B was increased in bronchial alveolar fluid (BAL) which is an indicator of recovery of alveolar epithelial function.
3.3.3.3. Umbilical cord derived mesenchymal stem cells
Chang et al. [52] in his study reported the case of a 59-year old man with a history of pulmonary tuberculosis and being treated with corticosteroids. After developing fever, cough and rhinorrhea, he was hospitalized and further developed progressive bilateral diffuse infiltrations. Finally, he was diagnosed with ARDS and hypoactive delirium because of his ill health. He was administered MSC intrathecally derived from umbilical cord which showed improvement in his laboratory parameters with a slight reduction in bilateral lung infiltrates. He further experienced 6 generalized tonic-clonic seizures for which he was put on an anti-convulsant. However, the patient could not survive due to repeated pulmonary infections but the study still suggests the possible use of MSCs in an ARDS patient.
3.4. Clinical trials registered
Registered Clinical Trials for the role of stem cell therapy in SARS-CoV-2 infection is shown in Table 4
Table 4.
List of registered stem cell therapy based clinical trials for COVID-19.
| Author, Year | Study design | Status | Population | Intervention/ Details of stem cell use (Stem size) | Control (sample size) |
Outcome |
|---|---|---|---|---|---|---|
| AlZoubi et al. 2020 [53] | Phase I Interventional | Recruiting | 5 Patients positively diagnosed with COVID-19 (18 years and older) | Wharton's Jelly-Mesenchymal stem cell | Primary Outcome: Improvement of clinical symptoms(fever, pneumonia, respiratory distress, sneezing, cough, diarrhea) | |
| Guillory et al. 2020 [54] | Phase 2 multi-center, double-blind, randomized, placebo-control clinical trial | Not yet recruiting | 200 (18 years and older) |
Autologous Adipose Tissue-Derived MSCs (Celltex-AdMSCs) | Phase 2 Placebo group | Primary Outcome: Safety and Tolerability Incidence of COVID-19 in study and control group |
| Akram et al. 2020 [55] | Prospective, Randomized Phase 2 Clinical Trial | Recruiting | 20 (10 years and older) |
MSCs | Phase 2 Placebo Group | Primary Outcome: Overall survival at 30 days post intervention |
| Q et al. 2020 [56] | Single-center, Prospective, Randomized Clinical Trial | Recruiting | 20 (18−65 years old) |
Allogeneic Human Dental Pulp Mesenchymal | Placebo group (3 mL of 0.9 % saline) | Primary Outcome: Time to Clinical Improvement |
| Vanegas et al. 2020 [57] | Phase 2 | Recruiting | 30 (18−79 years old) |
Umbilical cord derived MSCs | Placebo group | Primary Outcome: Clinical deterioration or death |
| Iglesias et al. 2020 [58] | Pilot Study | Recruiting | 10 (18 years and older) |
MSCs from umbilical cord allogenic | historical controls treated in INCMNSZ | Primary Outcome: clinical, biochemical, inflammatory and immune changes |
| Filho et al.2020 [59] | Phase 2 (Exploratory clinical study) | Recruiting | 90 (18 years and older) |
NestaCell® MSCs | Matching Placebo | Primary Outcome: change in clinical condition |
| Cheng et al.2020 [60] | Phase II, Randomized, Placebo-Controlled, Double-Blinded, Clinical Trial | Enrolling by invitation | 100 (Child, Adult, Older Adult) |
allogeneic adipose-derived MSCs | Placebo (Saline) | Primary Outcome: Incidence of hospitalization and symptoms associated with COVID-19 |
| Perez et al. 2020 [61] | Pilot phase, open label, non-randomized study | Active but not recruiting | 9 (18 years and older) |
MSCs derived from Wharton Jelly of Umbilical cords | Primary Outcome: Oxygen Saturation | |
| Gil et al. 2020 [62] | Phase I/II clinical trial | Recruiting | 26 (18–80 years old) |
Allogenic Adipose Tissue-Derived Mesenchymal | Control: No intervention | Primary Outcome: Safety and efficacy via ADR and survival rate |
| Cheng et al.2020 [63] | Phase II, Open Label, Single-Center, Clinical Trial | Enrolling by Invitation | 56 (Child, Adult, Older Adult) |
Autologous adipose-derived MSCs | Primary Outcome: Incidence of hospitalization and symptoms associated with COVID-19 | |
| Cheng et al. 2020 [64] | Open, single center, single arm test design | Not yet recruiting | 24 (18−75 years old) |
Dental pulp MSCs | Primary Outcome: Time of disappearance of ground-glass shadow in the lungs | |
| Shi et al. 2020 [65] | Phase 1 multicentric trial | Recruiting | 20 | Conventional treatment plus MSCs | Conventional Control Group | Primary Outcome: Evaluation of Pneumonia Improvement and Side-effects |
| Li et al. 2020 [66] | Phase 1/2 Randomized Controlled Trial | Not yet recruiting | 20 (18−75 years) |
BMMSCs | Conventional treatment plus placebo | Evaluation of pneumonia improvement and safety assessment |
| Wang et al. 2020 [67] | Phase II, Multicenter, Randomized, Double-blind, Placebo-controlled Trial | Active not recruiting | 100 (18−75 years) |
Human Umbilical Cord- MSCs | Standard of care plus 3 does of placebo | Evaluation of Pneumonia Improvement |
| Tarar et al. 2020 [68] | Phase 2 RCT | Recruiting | 20 (30−70 years) |
Umbilical Cord- MSCs | Treatment will be done for five subjects under Standard of Care | Assessment of Pneumonia and side effects. |
| Hancharou et al. 2020 [69] | Phase 1/2 | Enrolling by invitation | 40 (18−70 years) |
Allogenic pooled olfactory mucosa-derived mesenchymal | Standard Treatment | Number of patients cured |
| Liu et al. 2020 [70] | Phase 1/2 RCT | Recruiting | 30 (18−75 years) |
hMSC’s | Conventional treatment and Placebo intravenously | Improvement and recovery time of inflammatory and immune factors |
| Fujian et al. [71] 2020 | Early Phase 1 | Active not recruiting | 60 (18−70 years old) |
Umbilical Cord MSCs with Oseltamivir, hormone and oxygen therapy | oseltamivir, hormones, oxygen therapy, mechanical ventilation and other supportive therapies | Improvement of pulmonary function |
| Nouri et al. 2020 [72] | Phase 2−3 Clinical Trial | Recruiting | 60 18−65 years |
MSCs | Conventional therapy and supportive therapy | Evaluation of Pneumonia Improvement and safety assessment |
| [73] | Prospective Non-Interventional Study | Active, not recruiting | 40 3 Months and older |
Allogeneic Haematopoietic Stem Cell Transplantation | Comparison of inflammatory/immunological biomarkers | |
| Ricordi et al. 2020 [74] | Phase1/2 double blinded trial | Recruiting | 24 18 years and older |
Umbilical Cord Mesenchymal + Heparin along with best supportive care | Heparin + Supportive therapy | Assessment of safety |
| Thakur et al. 2020 [75] | Randomized, Placebo-Controlled, Double-Blind, Single Center, Phase 2 Trial | Not yet recruiting | 100 Child, Adult, Older Adult |
allogeneic adipose-derived MSCs | Placebo (Saline) | Detection of the changes from baseline for inflammatory markers, and oxygenation, and reduction in time for a negative PCR test result. |
| Adas et al. 2020 [76] | Prospective Double Controlled Study | Recruiting | 30 (40−60 years) |
MSCs | Patients that are on a ventilator and will receive saline injections | Covid-19 infection related symptom improvement. |
| Itticheria et al. 2020 [77] | Prospective, two-arm, partially masked, single center clinical study | Not yet recruiting | 20 18-60 years |
Stem cell Educator-Treated Mononuclear cells Apheresis | Conventional treatment of patients with SARS-CoV-2 | Patient number who could not complete the SCE therapy |
| [78] | NA | Withdrawn | 0 18−75 years |
hUC-MSC | ---- | ----- |
| Liao et al. 2020 [79] | Phase 1 Study | Not yet recruiting | 9 18-80 years old |
hUC-MSC (BX-U001) + supportive care | Assessment of safety | |
| Juan et al. 2020 [80] | Two-treatment,Randomized, Controlled, Multicenter Clinical Trial | Not yet recruiting | 100 | Allogeneic and expanded adipose tissue-derived MSCs | Regular respiratory distress treatment | Efficacy and safety assessment |
| Rosenberger et al. 2020 [81] | Prospective Phase II Study | Not yet recruiting | 40 18 and above |
MSCs in Inflammation-Resolution Programs of SARS-CoV-2 Induced ARDS | No intervention | Improvement of lung injury score |
| Qi et al. 2020 [82] | Phase 1 and 2 | Recruiting | 9 18−70 years |
Human Embryonic Stem Cells Derived M cells (CAStem) | Safety and efficacy | |
| Gelinjns et al. 2020 [83] | Randomized Phase 3 clinical trial | Recruiting | 300 18 years and older |
MSCs (Remestemcel-L) | Placebo | Number of all-cause mortality |
| Coll et al. 2020 [84] | Prospective, Double-blind, Randomized, Parallel, Placebo-controlled Pilot Clinical Trial | Recruiting | 30 18−75 years old |
XCEL-UMC-BETA (WJ-MSC) | Placebo | Number of patients who died |
| Stewart et al. 2020 [85] | Phase 1, open label, dose-escalating and safety trial using a 3 + 3+3 design | Not yet recruiting | 9 18 years and older |
Mesenchymal Stromal cells | Participant number with Treatment-Related Adverse effects. | |
| Fairbairn et al. 2020 [86] | Phase 1/2 Randomized, Placebo-Controlled Trial | Not yet recruiting | 70 18-85 years |
ACT-20-MSC | Placebo (MEM-α) | Mortality at day 30 |
| Sender et al. 2020 [[87]] | Phase 1b Randomized, Double-Blind, Placebo-Controlled Study | Not yet recruiting | 45 18-80 years |
BM-Allo.MSC | Placebo (plasmalyte and human albumin) | Incidence of AEs, mortality and its cause and Number of ventilator-free days |
| Rave et al. 2020 [88] | Phase I/II Study of Human Placental Hematopoietic stem cells Derived Natural Killer cells | Recruiting | 86 18 years and older |
CYNK-001 | Number and severity of adverse events for Phase 1 and Time to Clearance of SARS-CoV-2 for Phase 2 | |
| Hill et al. 2020 [89] | Early Phase 1 | Not yet recruiting | 30 18 years and older |
Mesenchymal Stromal cells | Incidence of unexpected adverse events and Improved oxygen saturations ≥93 % | |
| Barbado et al. 2020 [90] | Double Blind, Placebo-controlled, Phase II Trial | Recruiting | 24 18 years and older |
Allogenic Mesenchymal Stromal cells MSV | Placebo | Proportion of patients who have achieved withdrawal of invasive mechanical ventilation |
| Wang et al. 2020 [91] | Phase 2 | Recruiting | 16 18-80 years |
UC-MSCs | Oxygenation index | |
| Monsel et al. 2020 [92] | Phase1/2 | Recruiting | 40 18 years and older |
umbilical cord Wharton's jelly-derived mesenchymal stromal cells | NaCl 0.9 % | Efficacy in respiration |
| [93] | Randomized, Double-Blind, Placebo-Controlled, Multicenter, Parallel-Group Phase II Study | Recruiting | 140 40-80 years |
allogeneic ex vivo expanded placental mesenchymal-like adherent stromal cells (PLX-PAD) | Placebo solution for injection | Number of ventilator free days |
| Ting et al. 2020 [94] | Phase 2/3 Study | Recruiting | 400 18-89 years old |
MultiStem | Placebo intravenous infusion |
Ventilator-Free Days. Safety and tolerability |
| Miller et al. 2020 [95] | Multi-center, Randomized, Sham Controlled, Double-blind, Ascending-dose Study | Not yet recruiting | 24 18 years and older |
SBI-101 (device containing allogeneic hMSC’s + FDA-approved plasmapheresis device) | Sham device containing no MSCs | Safety and tolerability |
| Qu et al. 2020 (96) | Pilot Clinical Study | Not yet recruiting | 30 18−75 years |
Aerosol Inhalation of the Exosomes Derived from Allogenic Adipose Mesenchymal stem cells | Time to clinical improvement and safety |
4. Summary and conclusion
This systematic review yielded several important findings including in-vitro data of ARDS where stem cell therapy has been used, derived from various sources have shown increased proliferation and migration in lung cancer cell lines. They even exhibited decreased apoptosis and neutrophil migration. Similarly, in-vivo/preclinical data demonstrated the stem cells efficacy in different models of ALI. Results were quite promising as the stem cells demonstrated efficacious results in amelioration of lung fibrosis while decreasing the inflammation and edema. These findings very well correlated with certain case reports and clinical studies in terms of efficacy and safety profile where the capability of stem cell-based therapy to reduce the systemic inflammatory responses have been demonstrated in ARDS patients.
Additionally, these studies have highlighted several different mechanisms highlighting the importance of stem cells in restoration of the lung function. So, this published data of stem cells use in virus induced ARDS indicates a possible role of stem cells-based therapies for COVID-19 infection. Some case reports also discuss the safety and efficacy of stem cells in SARS-CoV-2 specifically in critically ill patients when they become unresponsive to conventional therapy. The benefits which have been noticed from these studies includes decreased inflammation by downregulating the cytokine levels without causing any allergic reactions, hence indicating tolerability to the treatment. Therefore, we believe that the current evidence from in-vitro/in-vivo and clinical studies suggests a potential of MSCs to be used in the treatment of COVID-19 patients. Although the results of clinical study data are motivating, still it will be too early to forecast potential therapeutic role of MSCs in COVID-19. Hence, large-scale trials of MSC therapy should be conducted to validate their clinical efficacy.
Declaration of Competing Interest
None.
References
- 1.Weiss S.R. Forty years with coronaviruses. J. Exp. Med. [Internet] 2020;217(March (5)) doi: 10.1084/jem.20200537. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7103766/ 30 [cited 2021 Jan 9]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC . Centers for Disease Control and Prevention; 2020. Coronavirus Disease 2019 (COVID-19) [Internet]https://www.cdc.gov/coronavirus/2019-ncov/index.html [cited 2021 Jan 9]. Available from: [Google Scholar]
- 3.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;22(Febuary (10224)):565–574. doi: 10.1016/S0140-6736(20)30251-8. 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Y., Yang C., Xu X., Xu W., Liu S. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020;41(September(9)):1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;27(March (1)):1620. doi: 10.1038/s41467-020-15562-9. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J., Deng W., Li S., Yang X. Advances in research on ACE2 as a receptor for 2019-nCoV. Cell Mol. Life Sci. CMLS. 2020 doi: 10.1007/s00018-020-03611-x. August 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutta N.K., Mazumdar K., Gordy J.T. The nucleocapsid protein of SARS–CoV-2: a target for vaccine development. J. Virol. [Internet] 2020;(June 16) doi: 10.1128/JVI.00647-20. https://jvi.asm.org/content/94/13/e00647-20 [cited 2021 Jan 9];94(13). Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H., Liu S.-M., Yu X-H Tang S.-L., Tang C.-K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int. J. Antimicrob. Agents. 2020;55(May(5)) doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scoring systems for predicting mortality for severe patients with COVID-19 - EClinicalMedicine [Internet]. [cited 2021 Jan 9]. Available from: https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(20)30170-X/fulltext. [DOI] [PMC free article] [PubMed]
- 10.Pharmacologic Treatments for Coronavirus Disease (COVID-19): a review | clinical pharmacy and pharmacology | JAMA |. JAMA Network [Internet] 2019 https://jamanetwork.com/journals/jama/fullarticle/2764727 [cited 2021 Jan 9]. Available from: [Google Scholar]
- 11.Mahalmani V.M., Mahendru D., Semwal A., Kaur S., Kaur H., Sarma P., et al. COVID-19 pandemic: a review based on current evidence. Indian J. Pharmacol. 2020;1(March (2)):117. doi: 10.4103/ijp.IJP_310_20. 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J.-Y., Qiao K., Liu F., Wu B., Xu X., Jiao G.-Q., et al. Lung transplantation as therapeutic option in acute respiratory distress syndrome for coronavirus disease 2019-related pulmonary fibrosis. Chin Med. J. (Engl.) 2020;20(June (12)):1390–1396. doi: 10.1097/CM9.0000000000000839. 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han W., Zhu M., Chen J., Zhang J., Zhu S., Li T., et al. Lung transplantation for elderly patients with end-stage COVID-19 pneumonia. Ann. Surg. 2020;272(July(1)):e33–4. doi: 10.1097/SLA.0000000000003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biehl J.K., Russell B. Introduction to stem cell therapy. J. Cardiovasc. Nurs. 2009;24(April(2)):98–103. doi: 10.1097/JCN.0b013e318197a6a5. quiz 104–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volarevic V., Gazdic M., Simovic Markovic B., Jovicic N., Djonov V., Arsenijevic N. Mesenchymal stem cell-derived factors: immuno-modulatory effects and therapeutic potential. BioFactors Oxf. Engl. 2017;10(September (5)):633–644. doi: 10.1002/biof.1374. 43. [DOI] [PubMed] [Google Scholar]
- 16.Evidence for HIV-1 cure after CCR5Δ32/Δ32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: a case report - The Lancet HIV [Internet]. [cited 2021 Jan 9]. Available from: https://www.thelancet.com/journals/lanhiv/article/PIIS2352-3018(20)30069-2/fulltext. [DOI] [PMC free article] [PubMed]
- 17.Peng L., Xie D., Lin B.-L., Liu J., Zhu H., Xie C., et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatol. Baltim. Md. 2011;2(September (3)):820–828. doi: 10.1002/hep.24434. 54. [DOI] [PubMed] [Google Scholar]
- 18.Xiao K., Hou F., Huang X., Li B., Qian Z.R., Xie L. Mesenchymal stem cells: current clinical progress in ARDS and COVID-19. Stem Cell Res. Ther. 2020;22(July (1)):305. doi: 10.1186/s13287-020-01804-6. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du J., Li H., Lian J., Zhu X., Qiao L., Lin J. Stem cell therapy: a potential approach for treatment of influenza virus and coronavirus-induced acute lung injury. Stem Cell Res. Ther. 2020;24(May (1)):192. doi: 10.1186/s13287-020-01699-3. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey C.C., Zhong G., Huang I.-C., Farzan M. IFITM-family proteins: the cell’s first line of antiviral defense. Annu. Rev. Virol. 2014;1(November 1):261–283. doi: 10.1146/annurev-virology-031413-085537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan M.C.W., Kuok D.I.T., Leung C.Y.H., Hui K.P.Y., Valkenburg S.A., Lau E.H.Y., et al. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 2016;29(March (13)):3621–3626. doi: 10.1073/pnas.1601911113. 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perlman S., Dandekar A.A. Immunopathogenesis of coronavirus infections: implications for SARS. Nat. Rev. Immunol. 2005;5(December(12)):917–927. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. August. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang W., Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif [Internet] 2019 doi: 10.1111/cpr.12712. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6985662/ November 15 [cited 2021 Jan 9];53(1). Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;15(June (12)):747–754. doi: 10.1089/scd.2020.0080. 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atluri S., Manchikanti L., Hirsch J.A. Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically ill COVID-19 patients: the case for compassionate use. Pain Physician. 2020;23(March(2)):E71–83. [PubMed] [Google Scholar]
- 27.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., et al. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pati S., Gerber M.H., Menge T.D., Wataha K.A., Zhao Y., Baumgartner J.A., et al. Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0025171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masterson C., Devaney J., Horie S., O’Flynn L., Deedigan L., Elliman S., et al. Syndecan-2-positive, bone marrow-derived human mesenchymal stromal cells attenuate bacterial-induced acute lung injury and enhance resolution of ventilator-induced lung injury in rats. Anesthesiology. 2018;129(September(3)):502–516. doi: 10.1097/ALN.0000000000002327. [DOI] [PubMed] [Google Scholar]
- 30.Xiang B., Chen L., Wang X., Zhao Y., Wang Y., Xiang C. Transplantation of menstrual blood-derived mesenchymal stem cells promotes the repair of LPS-Induced acute lung injury. Int. J. Mol. Sci. 2017;27(March (4)) doi: 10.3390/ijms18040689. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VY-F Su, Lin C.-S., Hung S.-C., Yang K.-Y. Mesenchymal stem cell-conditioned medium induces neutrophil apoptosis associated with inhibition of the NF-κB pathway in endotoxin-induced acute lung injury. Int. J. Mol. Sci. 2019;20(May (9)) doi: 10.3390/ijms20092208. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VY-F Su, Chiou S.-H., Lin C.-S., Chen W.-C., Yu W-K Chen Y.-W., et al. Induced pluripotent stem cells reduce neutrophil chemotaxis via activating GRK2 in endotoxin-induced acute lung injury. Respirol Carlton Vic. 2017;22(August(6)):1156–1164. doi: 10.1111/resp.13053. [DOI] [PubMed] [Google Scholar]
- 33.Engineered human mesenchymal stem cells as new vaccine platform for COVID-19 | bioRxiv [Internet]. [cited 2021 Jan 9]. Available from: https://www.biorxiv.org/content/10.1101/2020.06.20.163030v1.
- 34.Hu S., Li J., Xu X., Liu A., He H., Xu J., et al. The hepatocyte growth factor-expressing character is required for mesenchymal stem cells to protect the lung injured by lipopolysaccharide in vivo. Stem Cell Res. Ther. 2016;29(April (1)):66. doi: 10.1186/s13287-016-0320-5. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X., Wu S., Tang L., Ma L., Wang F., Feng H., et al. Mesenchymal stem cells overexpressing heme oxygenase-1 ameliorate lipopolysaccharide-induced acute lung injury in rats. J. Cell. Physiol. 2019;234(May(5)):7301–7319. doi: 10.1002/jcp.27488. [DOI] [PubMed] [Google Scholar]
- 36.Li L., Dong L., Zhang J., Gao F., Hui J., Yan J. Mesenchymal stem cells with downregulated Hippo signaling attenuate lung injury in mice with lipopolysaccharideinduced acute respiratory distress syndrome. Int. J. Mol. Med. 2019;43(March(3)):1241–1252. doi: 10.3892/ijmm.2018.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang S., Danchuk S.D., Imhof K.M., Semon J.A., Scruggs B.A., Bonvillain R.W., et al. Comparison of the therapeutic effects of human and mouse adipose-derived stem cells in a murine model of lipopolysaccharide-induced acute lung injury. Stem Cell Res. Ther. 2013;29(January (1)):13. doi: 10.1186/scrt161. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devaney J., Horie S., Masterson C., Elliman S., Barry F., O’Brien T., et al. Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax. 2015;70(July(7)):625–635. doi: 10.1136/thoraxjnl-2015-206813. [DOI] [PubMed] [Google Scholar]
- 39.Shalaby S.M., El-Shal A.S., Abd-Allah S.H., Selim A.O., Selim S.A., Gouda Z.A., et al. Mesenchymal stromal cell injection protects against oxidative stress in Escherichia coli-induced acute lung injury in mice. Cytotherapy. 2014;16(June(6)):764–775. doi: 10.1016/j.jcyt.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Zhao F., Zhang Y.F., Liu Y.G., Zhou J.J., Li Z.K., Wu C.G., et al. Therapeutic effects of bone marrow-derived mesenchymal stem cells engraftment on bleomycin-induced lung injury in rats. Transplant. Proc. 2008;40(June(5)):1700–1705. doi: 10.1016/j.transproceed.2008.01.080. [DOI] [PubMed] [Google Scholar]
- 41.Wakayama H., Hashimoto N., Matsushita Y., Matsubara K., Yamamoto N., Hasegawa Y., et al. Factors secreted from dental pulp stem cells show multifaceted benefits for treating acute lung injury in mice. Cytotherapy. 2015;17(August(8)):1119–1129. doi: 10.1016/j.jcyt.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Kumamoto M., Nishiwaki T., Matsuo N., Kimura H., Matsushima K. Minimally cultured bone marrow mesenchymal stem cells ameliorate fibrotic lung injury. Eur. Respir. J. 2009;34(September(3)):740–748. doi: 10.1183/09031936.00128508. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y., Ding J., Ren S., Wang W., Yang Y., Li S., et al. Intravenous infusion of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res. Ther. 2020;27(May (1)):207. doi: 10.1186/s13287-020-01725-4. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells [Internet]. [cited 2021 Jan 9]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7402800/. [DOI] [PMC free article] [PubMed]
- 45.Peng H., Gong T., Huang X., Sun X., Luo H., Wang W., et al. A synergistic role of convalescent plasma and mesenchymal stem cells in the treatment of severely ill COVID-19 patients: a clinical case report. Stem Cell Res. Ther. 2020;16(July (1)):291. doi: 10.1186/s13287-020-01802-8. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh S., Chakravarty T., Chen P., Akhmerov A., Falk J., Friedman O., et al. Allogeneic cardiosphere-derived cells (CAP-1002) in critically ill COVID-19 patients: compassionate-use case series. Basic Res. Cardiol. 2020;12(May (4)):36. doi: 10.1007/s00395-020-0795-1. 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng G., Huang L., Tong H., Shu Q., Hu Y., Ge M., et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir. Res. 2014;(April 4) doi: 10.1186/1465-9921-15-39. 15:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson J.G., Liu K.D., Zhuo H., Caballero L., McMillan M., Fang X., et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir. Med. 2015;3(January(1)):24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matthay M.A., Calfee C.S., Zhuo H., Thompson B.T., Wilson J.G., Levitt J.E., et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir. Med. 2019;7(Febuary(2)):154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simonson O.E., Mougiakakos D., Heldring N., Bassi G., Johansson H.J., Dalén M., et al. In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. Stem Cells Transl. Med. 2015;4(October(10)):1199–1213. doi: 10.5966/sctm.2015-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang Y., Park Sh, Huh J.-W., Lim C.-M., Koh Y., Hong S.-B. Intratracheal administration of umbilical cord blood-derived mesenchymal stem cells in a patient with acute respiratory distress syndrome. J. Korean Med. Sci. 2014;29(March(3)):438–440. doi: 10.3346/jkms.2014.29.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zoubi A.A. 2020. Treatment of COVID-19 Patients Using Wharton’s Jelly-Mesenchymal Stem Cells [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04313322 March [cited 2021 Jan 7]. Report No.: NCT04313322. Available from: [Google Scholar]
- 53.Celltex Therapeutics Corporation . 2020. Clinical Study for the Prophylactic Efficacy of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells (AdMSCs) Against Coronavirus 2019 (COVID-19) [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04428801 November [cited 2021 Jan 7]. Report No.: NCT04428801. Available from: [Google Scholar]
- 54.Akram D.Z. 2020. Prospective, Randomized Phase 2 Clinical Trial of Mesenchymal Stem Cells(MSCs) for the Treatment of Coronavirus Disease 2019(COVID-19) [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04444271 June [cited 2021 Jan 7]. Report No.: NCT04444271. Available from: [Google Scholar]
- 55.Qingsong Y. Safety and Efficacy Study of Allogeneic Human Dental Pulp Mesenchymal Stem Cells to Treat Severe Pneumonia of COVID-19:a Single-center, Prospective, Randomised Clinical Trial [Internet]. clinicaltrials.gov; 2020 April [cited 2021 Jan 7]. Report No.: NCT04336254. Available from: https://clinicaltrials.gov/ct2/show/NCT04336254.
- 56.2020. Trustem. Safety and Efficacy of Mesenchymal Stem Cells in the Management of Severe COVID-19 [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04429763 June [cited 2021 Jan 7]. Report No.: NCT04429763. Available from: [Google Scholar]
- 57.Iglesias M. 2020. Mesenchymal Stem Cells for the Treatment of Severe Acute Respiratory Distress Syndrome Due to COVID-19. Pilot Study [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04416139 June [cited 2021 Jan 7]. Report No.: NCT04416139. Available from: [Google Scholar]
- 58.Brasil Azidus. 2020. Exploratory Clinical Study to Assess the Efficacy of NestaCell® Mesenchymal Stem Cell to Treat Patients with Severe COVID-19 Pneumonia [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04315987 June [cited 2021 Jan 7]. Report No.: NCT04315987. Available from: [Google Scholar]
- 59.Biosciences Hope. 2020. A Randomized, Double-Blind, Single Center, Efficacy and Safety Study of Allogeneic HB-adMSCs to Provide Immune Support against COVID-19 [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04348435 July [cited 2021 Jan 7]. Report No.: NCT04348435. Available from: [Google Scholar]
- 60.Instituto de Medicina Regenerativa . 2020. A Study of Mesenchymal Stem Cells as a Treatment in Patients with Acute Respiratory Distress Syndrome Caused by COVID-19 [Internet]. clinicaltrials.gOv.https://clinicaltrials.gov/ct2/show/NCT04456361 August [cited 2021 Jan 7]. Report No.: NCT04456361. Available from: [Google Scholar]
- 61.Andalusian Network for Design and Translation of Advanced Therapies . 2020. Phase I / II Clinical Trial, Multicenter, Randomized and Controlled, to Assess the Safety and Efficacy of Intravenous Administration of Allogeneic Adult Mesenchymal Stem Cells of Expanded Adipose Tissue in Patients With Severe Pneumonia Due to COVID-19 [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04366323 November [cited 2021 Jan 7]. Report No.: NCT04366323. Available from: [Google Scholar]
- 62.Biosciences Hope. 2020. A Phase II, Open Label, Single-Center, Clinical Trial to Assess Efficacy of HB-adMSCs to Provide Immune Support against Coronavirus Disease [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04349631 October [cited 2021 Jan 7]. Report No.: NCT04349631. Available from: [Google Scholar]
- 63.2020. CAR-T (Shanghai) Biotechnology Co., Ltd. Clinical Study of Novel Coronavirus Induced Severe Pneumonia Treated by Dental Pulp Mesenchymal Stem Cells [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04302519 March [cited 2021 Jan 7]. Report No.: NCT04302519. Available from: [Google Scholar]
- 64.Wang F.-S. 2020. Safety and Efficiency of Mesenchymal Stem Cell in Treating Pneumonia Patients Infected With COVID-19 [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04252118 April [cited 2021 Jan 7]. Report No.: NCT04252118. Available from: [Google Scholar]
- 65.Li S. 2020. Safety and Efficacy of Intravenous Infusion of Bone Marrow-Derived Mesenchymal Stem Cells in Severe Patients with Coronavirus Disease 2019 (COVID-19): A Phase 1/2 Randomized Controlled Trial [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04346368 Apr [cited 2021 Jan 7]. Report No.: NCT04346368. Available from: [Google Scholar]
- 66.Wang F.-S.A. 2020. Phase II, Multicenter, Randomized, Double-blind, Placebo-controlled Trial to Evaluate the Efficacy and Safety of Human Umbilical Cord-derived Mesenchymal Stem Cells in the Treatment of Severe COVID-19 Patients [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04288102 August [cited 2021 Jan 7]. Report No.: NCT04288102. Available from: [Google Scholar]
- 67.Riazuddin P.S. 2020. Efficacy of Intravenous Infusions of Stem Cells in the Treatment of COVID-19 Patients [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04437823 June [cited 2021 Jan 7]. Report No.: NCT04437823. Available from: [Google Scholar]
- 68.Institute of Biophysics and Cell Engineering of National Academy of Sciences of Belarus . 2020. Treatment of Covid-19 Associated Pneumonia With Allogenic Pooled Olfactory Mucosa-derived Mesenchymal Stem Cells [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04382547 May [cited 2021 Jan 7]. Report No.: NCT04382547. Available from: [Google Scholar]
- 69.Puren Hospital Affiliated to Wuhan University of Science and Technology . 2020. Clinical Research of Human Mesenchymal Stem Cells in the Treatment of COVID-19 Pneumonia [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04339660 April [cited 2021 Jan 7]. Report No.: NCT04339660. Available from: [Google Scholar]
- 70.Wang F.-S. 2020. Safety and Efficiency of Mesenchymal Stem Cell in Treating Pneumonia Patients Infected With COVID-19 [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04252118 April [cited 2021 Jan 7]. Report No.: NCT04252118. Available from: [Google Scholar]
- 71.Great Ormond Street Hospital for Children NHS Foundation Trust . 2020. A Prospective Non Interventional Study to Evaluate the Role of Immune and Inflammatory Response in Recipients of Allogeneic Haematopoietic Stem Cell Transplantation (SCT) Affected by Severe COVID19 Infection [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04349540 April [cited 2021 Jan 7]. Report No.: NCT04349540. Available from: [Google Scholar]
- 72.Ricordi C. 2020. Umbilical Cord-derived Mesenchymal Stem Cells for COVID-19 Patients with Acute Respiratory Distress Syndrome (ARDS) [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04355728 November [cited 2021 Jan 7]. Report No.: NCT04355728. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Biosciences Hope. 2020. A Randomized, Placebo-Controlled, Double-Blind, Efficacy and Safety Study of Allogeneic HB-adMSCs for the Treatment of COVID-19 [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04362189 December [cited 2021 Jan 7]. Report No.: NCT04362189. Available from: [Google Scholar]
- 74.Dr S.B.Ü. 2020. Sadi Konuk Eğitim Ve Araştırma Hastanesi. What Is the Effect of Mesenchymal Stem Cell Therapy on Seriously Ill Patients With Covid 19 in Intensive Care? (Prospective Double Controlled Study) [Internet]. clinicaltrials.gOv.https://clinicaltrials.gov/ct2/show/NCT04392778 May [cited 2021 Jan 7]. Report No.: NCT04392778. Available from: [Google Scholar]
- 75.Tianhe Stem Cell Biotechnologies Inc . 2020. Clinical Application of Stem Cell Educator Therapy for the Treatment of Viral Inflammation Caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04299152 October [cited 2021 Jan 7]. Report No.: NCT04299152. Available from: [Google Scholar]
- 76.Baylx Inc . 2020. A Phase 1 Randomized, Placebo-Controlled, Double-Blind Study of the Safety and Tolerability of a Single Intravenous Infusion of BX-U001, a Human Umbilical Cord Tissue Derived Mesenchymal Stem Cell Product, for Refractory Rheumatoid Arthritis [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT03828344 June [cited 2021 Jan 7]. Report No.: NCT03828344. Available from: [Google Scholar]
- 77.Instituto de Investigación Sanitaria de la Fundación Jiménez Díaz . 2020. Two-treatment,Randomized, Controlled, Multicenter Clinical Trial to Assess the Safety and Efficacy of Intravenous Administration of Expanded Allogeneic Adipose Tissue Adult Mesenchymal Stromal Cells in Critically Ill Patients COVID-19 [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04348461 April [cited 2021 Jan 7]. Report No.: NCT04348461. Available from: [Google Scholar]
- 78.University Hospital Tuebingen . 2020. Prospective Phase II Study: MSCs in Inflammation-Resolution Programs of SARS-CoV-2 Induced ARDS [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04377334 June [cited 2021 Jan 7]. Report No.: NCT04377334. Available from: [Google Scholar]
- 79.Zhou Q. 2020. Safety and Efficacy Study of Human Embryonic Stem Cells Derived M Cells (CAStem) for the Treatment of Severe COVID-19 Associated With or Without Acute Respiratory Distress Syndrome (ARDS) [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04331613 March [cited 2021 Jan 7]. Report No.: NCT04331613. Available from: [Google Scholar]
- 80.University Hospital Tuebingen . 2020. Prospective Phase II Study: MSCs in Inflammation-Resolution Programs of SARS-CoV-2 Induced ARDS [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04377334 June [cited 2021 Jan 7]. Report No.: NCT04377334. Available from: [Google Scholar]
- 81.Zhou Q. 2020. Safety and Efficacy Study of Human Embryonic Stem Cells Derived M Cells (CAStem) for the Treatment of Severe COVID-19 Associated With or Without Acute Respiratory Distress Syndrome (ARDS) [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04331613 March [cited 2021 Jan 7]. Report No.: NCT04331613. Available from: [Google Scholar]
- 82.Gelijns A. 2020. Mesenchymal Stromal Cells for the Treatment of Moderate to Severe COVID-19 Acute Respiratory Distress Syndrome [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04371393 December [cited 2021 Jan 7]. Report No.: NCT04371393. Available from: [Google Scholar]
- 83.Banc de Sang i Teixits . 2020. A Prospective, Double-blind, Randomized, Parallel, Placebo-controlled Pilot Clinical Trial for the Evaluation of the Efficacy and Safety of Two Doses of WJ-MSC in Patients With Acute Respiratory Distress Syndrome Secondary to Infection by COVID-19 [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04390139 May [cited 2021 Jan 7]. Report No.: NCT04390139. Available from: [Google Scholar]
- 84.Ottawa Hospital Research Institute Cellular Immuno-Therapy for COVID-19 ARDS (CIRCA-19) the Vanguard Study [Internet]. clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04400032 2020 September [cited 2021 Jan 7]. Report No.: NCT04400032. Available from:
- 85.Aspire Health Science . 2020. A Phase 1/2 Randomized, Placebo-Controlled Trial of ACT-20 in Patients with Severe COVID-19 Pneumonia [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04398303 May [cited 2021 Jan 7]. Report No.: NCT04398303. Available from: [Google Scholar]
- 86.NantKwest Announces FDA Authorization of I.N.D. Application for Mesenchymal Stem Cell Product for the Treatment of Severe COVID-19 Patients -. NantKwest [Internet]. [cited 2021 Jan 9]. Available from: https://nantkwest.com/nantkwest-announces-fda-authorization-of-ind-application-for-mesenchymal-stem-cell-product-for-the-treatment-of-severe-covid-19-patients/.
- 87.Celularity Incorporated . 2020. A Phase I/II Study of Human Placental Hematopoietic Stem Cell Derived Natural Killer Cells (CYNK-001) for the Treatment of Adults With COVID-19 [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04365101 November [cited 2021 Jan 7]. Report No.: NCT04365101. Available from: [Google Scholar]
- 88.Hill L. 2020. Single Donor Banked Bone Marrow Mesenchymal Stromal Cells for the Treatment of COVID19-Induced ARDS: A Randomized, Controlled Study [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04345601 November [cited 2021 Jan 7]. Report No.: NCT04345601. Available from: [Google Scholar]
- 89.Red de Terapia Celular . 2020. Double Blind, Placebo-controlled, Phase II Trial to Evaluate Safety and Efficacy of Allogenic Mesenchymal Stromal Cells MSV_allo for Treatment of Acute Respiratory Failure in Patients With COVID-19 Pneumonia (COVID_MSV) [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04361942 October [cited 2021 Jan 7]. Report No.: NCT04361942. Available from: [Google Scholar]
- 90.Peng Z. 2020. Clinical Research Regarding the Availability and Safety of UC-MSCs Treatment for Serious Pneumonia and Critical Pneumonia Caused by the 2019-nCOV Infection [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04269525 June [cited 2021 Jan 7]. Report No.: NCT04269525. Available from: [Google Scholar]
- 91.Assistance Publique - Hôpitaux de Paris . 2020. Cell Therapy Using Umbilical Cord-derived Mesenchymal Stromal Cells in SARS-CoV-2-related ARDS [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04333368 December [cited 2021 Jan 7]. Report No.: NCT04333368. Available from: [Google Scholar]
- 92.Pluristem Ltd . 2020. Efficacy, Tolerability and Safety of Intramuscular Injections of PLX PAD for the Treatment of Subjects With Critical Limb Ischemia (CLI) With Minor Tissue Loss Who Are Unsuitable for Revascularization [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT03006770 December [cited 2021 Jan 7]. Report No.: NCT03006770. Available from: [Google Scholar]
- 93.Athersys, Inc . 2020. A Phase 2/3 Study to Assess the Safety and Efficacy of MultiStem® Therapy in Subjects with Acute Respiratory Distress Syndrome (ARDS) Due to Coronavirus Disease (COVID-19) [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04367077 September [cited 2021 Jan 7]. Report No.: NCT04367077. Available from: [Google Scholar]
- 94.Sentien Biotechnologies, Inc . 2020. A Multi-center, Randomized, Case Controlled, Double-blind, Ascending-dose Study of Extracorporeal Mesenchymal Stromal Cell Therapy (SBI-101 Therapy) in COVID-19 Subjects with Acute Kidney Injury Receiving Renal Replacement Therapy [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04445220 November [cited 2021 Jan 7]. Report No.: NCT04445220. Available from: [Google Scholar]
- 95.Hospital Ruijin. 2020. A Pilot Clinical Study on Aerosol Inhalation of the Exosomes Derived From Allogenic Adipose Mesenchymal Stem Cells in the Treatment of Severe Patients With Novel Coronavirus Pneumonia [Internet]. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT04276987 September [cited 2021 Jan 7]. Report No.: NCT04276987. Available from: [Google Scholar]