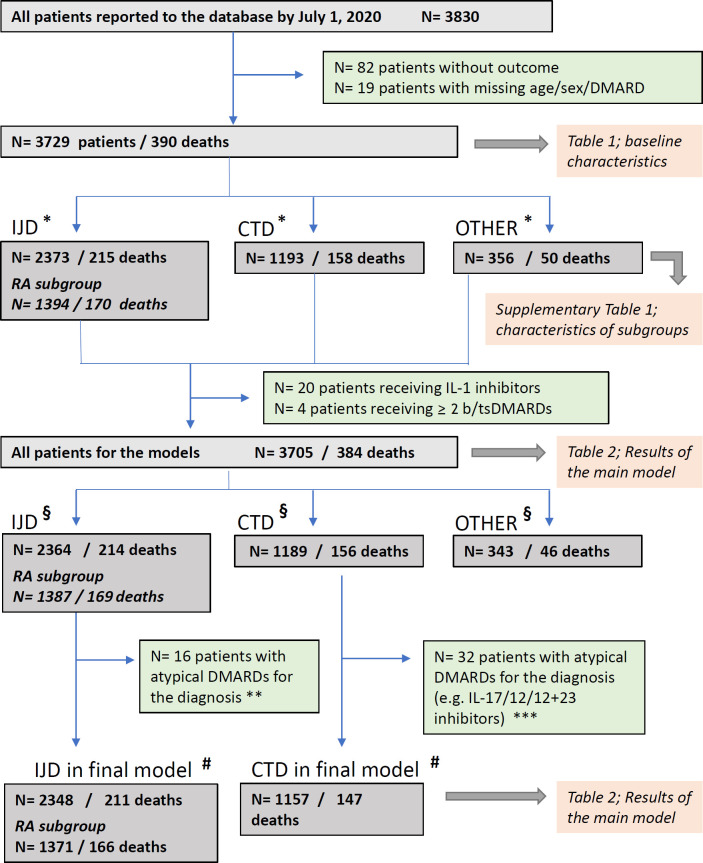
Figure 2.
Patient flowchart. Some patients had diagnoses in multiple groups; as a result, the sum of patients in each group is greater than the total number of patients. (*) Patients belonging to more than one diagnosic group: IJD and CTD: N=78 (10 deaths); IJD and other: N=70 (12 deaths); CTD and other: N=50 (13 deaths); IJD and CTD and other: N=5 (2 deaths). (§) Patients belonging to more than one diagnosic group: IJD and CTD: N=77 (10 deaths); IJD and other: N=70 (12 deaths); CTD and other: N=49 (12 deaths); IJD and CTD and other: N=5 (2 deaths). (#) Patients belonging to more than one diagnosic group: IJD and CTD: N=59 (7 deaths). (**) Non-typical DMARDs for IJD and RA: immunosuppressants and belimumab; non-typical DMARDs for RA: IL-17/IL-23/IL-12+23 inhibitors. (***) Non-typical DMARDs for CTD: abatacept, IL-17/IL-23/IL-12+23 inhibitors, sulfasalazine, leflunomide and tsDMARDs. b/tsDMARDs, biological/targeted synthetic disease-modifying antirheumatic drugs; CTD, connective tissue disease/vasculitis; DMARDs, disease-modifying anti-rheumatic drugs; IJD, inflammatory joint disease; IL, interleukin; RA, rheumatoid arthritis.