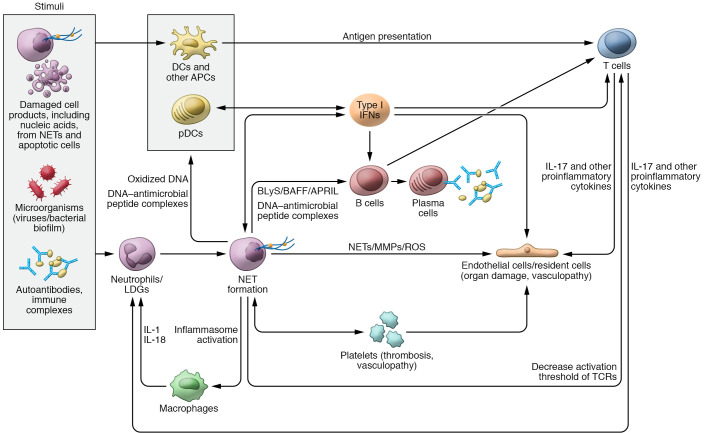
Figure 1. Role of neutrophils, NETs, and IFNs in SLE pathogenesis.
Various stimuli can trigger neutrophils to undergo neutrophil extracellular trap (NET) formation. NETs, in turn, can externalize self-antigens, including oxidized DNA and/or DNA–antimicrobial peptide complexes that can be presented to antigen-presenting cells (APCs) and activate plasmacytoid DCs (pDCs) to synthesize type I IFNs. NETs have the ability to activate the NLRP3 inflammasome in macrophages, resulting in increased release of IL-1 and IL-18, which further prime neutrophils to undergo NET formation and perpetuate tissue damage. Different exogenous and endogenous stimuli can promote type I IFN generation. The synthesis of type I IFNs further modulates other APCs, tissue-resident cells, and T and B cell functions. NET products and IFNs modulate T cell responses and can also activate B cells to undergo class switching and secrete autoantibodies against a wide range of self-antigens. DNA–antimicrobial peptide complexes (like LL37-DNA) released from NETs have the ability to directly activate B cells via TLR9 and promote autoantibody generation. NETs directly stimulate T cells by decreasing their activation threshold via Zap70-mediated phosphorylation of the T cell receptor (TCR). Activated T cells release IL-17 and other proinflammatory cytokines that can result in endothelial cell damage as well prime neutrophils to undergo further NET formation and migrate to inflamed tissues. NETs and IFNs can promote direct tissue damage and vascular inflammation through their effect on endothelial cells and platelets. APRIL, a proliferation-inducing ligand; BAFF, B cell activating factor; BLyS, B lymphocyte stimulator; LDG, low-density granulocyte.