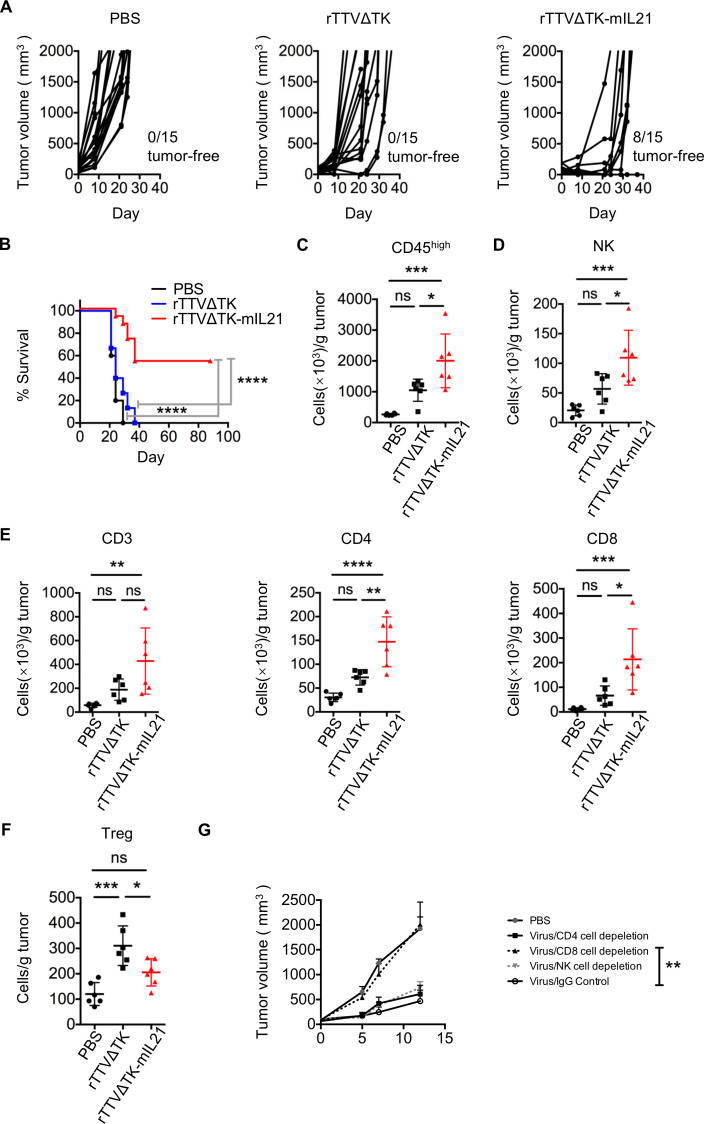
Figure 3.
Introduction of IL-21 into rTTVΔTK significantly enhanced its tumor-suppressing activity. B16 cells (5×104) were subcutaneously implanted into the right flank of mice, tumors were allowed to reach approximately 50 mm3 and then subjected to intratumoral injection with PBS, TTVΔTK (1×107 PFU) or rTTVΔTK-mIL21 (1×107 PFU). (A) Tumor growth of individual injected tumors with ratios of tumor-free mice after treatment indicated (n=15 per group). (B) Cumulative survival curves (****p<0.0001, log-rank test). Data are representative of three independent experiments. (C–F) Tumor infiltration of immune effector cells. Tumors were established in mice by implantation of 1×105 B16 cells into the right flank, followed by intratumoral injection of PBS, TTVΔTK (1×107 PFU) or rTTVΔTK-mIL21 (1×107 PFU). Tumors were isolated 4 days later to analyze the levels of infiltrating immune cells by flow cytometry. The abundances of tumor-infiltrating immune cells and their subpopulations are shown in (C) CD45high, (D) NK, (E) CD3+, CD4+, CD8+ and (F) Tregs (n=6, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, NS: p>0.05, one-way ANOVA). Data are representative of three independent experiments. (G) Assessment of individual contribution of NK cells, CD4+ cells and CD8+ cells to the rTTVΔTK-mIL21–mediated control of tumor growth. Mice were treated as described above except that intraperitoneal administrations of cell-depleting antibody or control IgG antibodies were applied, beginning a day prior to the virus injection and continuing twice weekly throughout the experiment (n=6, **p<0.01 by one-way ANOVA; mean±SEM is shown).