Abstract
The topic of interstitial pneumonia with autoimmune features (IPAF) is a research classification proposed by the European Respiratory Society/American Thoracic Society Task Force: this includes patients with idiopathic interstitial pneumonia (IIP) and clinical features, suggesting an underlying autoimmune process, but who do not meet established criteria for a connective tissue disease (CTD). We aimed to perform a detailed characterization of clinical, serological, and radiological features for our patients with IPAF criteria. Six patients were included, and a comprehensive description of these cases revealed a heterogeneous group in terms of clinical and treatment options. In most patients, it was possible to identify other features and disorders with an autoimmune “background,” which may support the inclusion of these patients in the IPAF classification. No deaths or significant decline in lung function occurred, and thus no definitive diagnosis of CTD could be found over 35 months of median follow-up. Therefore, IPAF is a recent concept, with many questions still open in regard to its usage in the ILD field.
Keywords: Interstitial pneumonia with autoimmune features, Interstitial lung disease, Connective tissue disease
Highlights
-
•
IPAF allows to group who presents with IIP and features suggesting an underlying autoimmune process, but do not meet criteria for a CTD.
-
•
A detailed clinical history is crucial to identify other features or disorders with an autoimmune “background”.
-
•
Our cases reveal a heterogeneous population, with a “benign” disease behavior, similar to CTD-ILD.
-
•
The multidisciplinary meeting and follow-up are essential, so that an IPF diagnosis (if UIP pattern is present), or an evolution to CTD-ILD, are not missed.
1. Introduction
Many patients with an idiopathic interstitial pneumonia (IIP) have clinical features that suggest an underlying autoimmune process, but who do not meet established criteria for a connective tissue disease (CTD) [1]. Previously, patients were labeled as undifferentiated CTD-associated interstitial lung disease (ILD) (UCTD-ILD), lung-dominant CTD, or autoimmune-featured ILD (AI-ILD) by study groups using overlapping, but slightly different criteria [[2], [3], [4]].
Recently, the term “interstitial pneumonia with autoimmune features” (IPAF) was proposed by the European Respiratory Society (ERS)/American Thoracic Society (ATS) task force to group these patients [5]. This new classification system incorporates clinical and serological domains, as well as morphological features encountered in high-resolution computed tomography (HRCT), surgical lung biopsy (SLB), and pulmonary function testing (PFTs). This paper's intent was to identify individuals with IIP and related suggestive features, but not in a definitive manner for a CTD; therefore, we hope to promote understanding and facilitate future research in the ILD field.
As previously reported with other ILDs, it appears to exist as a significant heterogeneity within the IPAF phenotype, which may have prognostic implications [6,7]. The impact of the introduction of the IPAF term in the ILD classification is still under discussion: many patients appear to have an intermediate prognosis, regarding mortality between CTD-ILD and idiopathic pulmonary fibrosis (IPF) [8]. However, these data were not confirmed by Ahmad et al., who reported a similar prognosis for IPAF and IPF patients, despite the disease's imaging pattern [9].
Nevertheless, most studies in the literature about IPAF are retrospective, descriptive, along with methodological limitations (selection or referral bias). Recently, Sambataro et al. described for the first time the clinical, serological, and radiological features of a prospective cohort of IPAF patients. In comparison to IPF, IPAF patients show a female predominance, younger age, better performance in PFTs, and less necessity for O2 support [10].
We analyzed our patients followed in the ILD outpatient department, and identified those who fulfilled the diagnostic criteria of IPAF. A detailed characterization of clinical, serological and radiological features of them was performed utilizing a chart review. All patients were discussed at the ILD multidisciplinary meeting and were evaluated by an autoimmune specialist.
2. Case descriptions
The authors identified six cases of IPAF. Detailed demographic and clinical characterizations of these patients is demonstrated in Table 1.
Table 1.
Patient's demographic and clinical characteristics.
| Variables | N (%)/Median (max-min value) |
|---|---|
| Female gender | 5/6 (83.3) |
| Age (years) | 66 (56–85) |
| Smoking status Non-smoker | Former smoker |
5/6 (83.3) | 1/6 (16.7) |
| Oxygenotherapy | 2/6 (33.3) |
| Symptoms | |
| No respiratory symptoms | 1/6 |
| Dyspnea | 5/6 |
| Cough | 4/6 |
| Constitutional symptoms | 3/6 |
| Chest pain | 0/6 |
| Bronchoalveolar lavage (BAL) (%) | |
| Lymphocytes | 16.8 (11.2–24) |
| Neutrophils | 9.8 (3.2–13.0) |
| Eosinophils | 5.0 (0–9.8) |
| Lung Function at diagnosis (%predicted) | |
| Forced Vital Capacity (FVC) | 103.0 (55–131) |
| Forced Expiratory volume in 1st second (FEV1) | 111.5(60–145) |
| Total Lung Capacity (TLC) | 96.5 (62–115) |
| Carbon Monoxide Diffusion Capacity (DLCO) | 51 (21–56) |
| Treatment | 5/6 |
| Corticosteroids + azathioprine | 2/6 |
| Corticosteroids + hydroxychloroquine | 1/6 |
| Mofetil mycophenolate | 1/6 |
| Corticosteroids | 1/6 |
According to Fisher et al. criteria [5], three patients met all the diagnostic domains, two met both serological and morphological domains, and one met clinical and serological criteria (Table 2).
Table 2.
Description of IPAF domains, other autoimmune diseases or features suggestive of autoimmunity.
| ♀Case 1 | ♀Case 2 | ♀Case 3 | ♀Case 4 | ♀Case 5 | ♂Case 6 | |
|---|---|---|---|---|---|---|
| Clinical domain | – | Inflammatory arthritis; unexplained digital oedema | Raynaud's phenomenon | Inflammatory arthritis; unexplained digital oedema | – | Inflammatory arthritis |
| Serologic domain | ANA≥1:320, speckled pattern | Rheumatoid factor ≥2x LSN | ANA≥1:320 speckled pattern |
ANA nucleolar | ANA nucleolar pattern Anti-dsDNA Anti-CPA |
ANAs ≥1:320 |
| Morphologic domain | NSIP | overlap NSIP + OP | (UIP) Unexplained pericardial effusion |
NSIP | OP | (UIP) |
| Other autoimmune diseases | Autoimmune thyroiditis |
– | Autoimmune hepatitis | Autoimmune thyroiditis Thrombotic thrombocytopenic purpura |
– | Inflammatory bowel disease |
| Other features suggestive of autoimmunity | Esophageal dysmotility | Anti-mitochondrial antibodies | – | Anti-parietal cells antibodies | – | Familiar history of autoimmune disease |
(ANA = antinuclear antibody; NSIP = non-specific interstitial pneumonia; OP = organizing pneumonia; UIP = usual interstitial pneumonia; anti-dsDNA = Anti-double stranded DNA; anti-CPA = Anti-citrullinated protein antibodies).
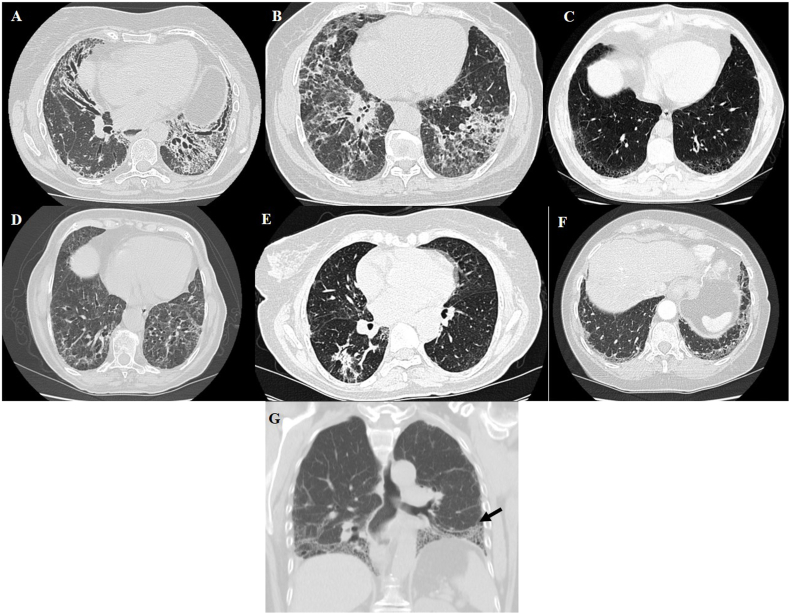
Concerning clinical domains, three patients presented inflammatory arthritis, two unexplained digital oedema, and one had Raynaud's disease. Considering the serologic domain, three patients had an antinuclear antibody (ANA) titer ≥1:320, two an ANA nucleolar pattern, and one a rheumatoid factor greater than twice the normal upper limit. In the morphological domain, the most frequent HRCT pattern was nonspecific interstitial pneumonia (NSIP) (2/6), followed by NSIP with an OP overlap (1/6), and organizing pneumonia (OP) (1/6). Patients 3 and 6 had the usual interstitial pneumonia (UIP) patterns for HRCT (Fig. 1). Patient 5 had histological confirmation of organizing pneumonia with CT-guided transthoracic lung biopsy. Unexplained pericardial effusion was the only other multicompartment finding as seen in these patients (Patient 3).
Fig. 1.
–Radiological features of IPAF patients. (A) Fibrotic non-specific interstitial pneumonia (NSIP) with traction bronchiectasis – patient one; (B) Fibrotic non-specific interstitial pneumonia (NSIP) + Organizing pneumonia pattern – patient two; (C) Usual interstitial pneumonia pattern – patient three; (D) Fibrotic non-specific interstitial pneumonia (NSIP) – patient four; (E) Organizing pneumonia pattern – patient five; (F) Honeycombing and ground-glass on the lung bases – patient six (G) “Straight edge” sign – isolation of fibrosis to the lung bases with sharp demarcation in the craniocaudal plane without substantial extension along the lateral margins of the lungs on coronal images, more typical of ILD-CTD [13,14]. - patient one.
Other autoimmune diseases were present: autoimmune thyroiditis (2/6), inflammatory bowel disease (1/6), idiopathic thrombocytopenic purpura (1/6), and autoimmune hepatitis (1/6). Some autoimmune features were described, such as esophageal dysmotility (1/6), antibodies not included in the IPAF criteria (2/6), and a positive family history of autoimmune disease (1/6). (Table 2).
Most patients were treated with corticosteroids and/or an immunosuppressant. Patient two presented disease progression, despite azathioprine treatment and was initiated on mofetil mycophenolate. Patient six was treated with vedolizumab for inflammatory bowel disease, without treatment for ILD. No side effects were reported in our patients (Table 1).
No significant decline in lung function (forced vital capacity (FVC) or diffusing capacity for carbon monoxide (DLCO)) were verified during follow-up. The baseline median FVC of our patients was 103% of what was predicted (55%–131% of predicted) and the median DLCO was 51% of predicted (21%–56% of predicted). At one-year follow-up, the median FVC was 106% of predicted (58%-126% of predicted) and median DLCO was 49% of predicted (28%–85% of predicted).
No new clinical, serological, or morphological criteria for CTD were identified during a median follow-up of 35 months (15–48 months).
3. Discussion
A such, the description of these cases that fulfill the IPAF criteria reveal a heterogeneous group with female predominance, a median age of around 65 years-old, and mostly non-smokers, as previously published in the literature [[8], [9], [10], [11], [12]]. The clinical characteristics, outcomes, and treatment options reported in the preceding IPAF series are summarized in Table 3.
Table 3.
Patient clinical characteristics and Interstitial Pneumonia with Autoimmune features (IPAF) domains reported in previous studies. (* - prospective study).
| Patients | Demographic | UIP | Outcome | IPAF domains | Clinical (most frequent) |
Serological (most frequent) | Morphological (most frequent) |
Treatment | Other findings | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sambataro et al. [10] * | 45 | Female (62.2%) Median age 66 (59.5–71.0) Non-smoker (48.9%) O2 usage 44.4% |
17.8% | Death NR Lung transplant NR | C + S 0% C + M 51.1% S + M 37.8% C + S + M 11.1% |
Raynaud's phenomenon 31.1% Arthritis 26.7% Unexplained digital oedema 8.9% |
ANA 17.8% RF 15.6% Anti-CPA 6.7 |
NSIP 68.9% OP 4.4% NSIP + OP 2.2% |
NR | IPAF patients seem to have a less severe lung disease and need of oxygen therapy than IPF. |
| Oldham et al. [8] | 144 | Female (52.1%) Mean age 63 ± 11 Non-smoker (54.9%) O2 usage NR |
54.6% | Death 39.6% Lung transplant 10.3% |
C + S 14.6% C + M 8.3% S + M 50.7% C + S + M 26.4% |
Raynaud's phenomenon 27.8% |
ANA 77.6% Anti-SSA 16.6% RF 13% |
NSIP 31.9 NSIP + OP 7.8% OP 3.5% |
CCT 32.2% IS NR |
IPAF better survival than IPF; worse than CDT-ILD; UIP associated with worse survival. |
| Ahmad et al. [9] | 57 | Female (49.1%) Mean age 64.4 ± 14.0 Non-smoker (34.0%) O2 usage NR |
28% | Death 12.2% Lung transplant NR |
C + S 11.1% C + M 7.0% S + M 52.7% C + S + M 29.2% |
Raynaud's phenomenon 35.0% Arthritis 48.1% Palmar telangiectasia 25.9% |
ANA 82.4% Anti-tRNA 17.0% Anti-CCP 9.4% |
NSIP 42.1% NSIP + OP 15.8% OP 3.5% |
CCT 67.9% IS 28.6% Antifibrotic 5.4% |
No significant difference was found in overall survival between IPAF and IPF. |
| Chartrand et al. [11] | 56 | Female (71.4%) Mean age 56.6 ± 10.3 Non-smoker (32.2%) O2 usage NR |
8.9% | Death 0% Lung transplant NR |
C + S 1.5% C + M 9.0% S + M 37.5% C + S + M 52.0% |
Raynaud's phenomenon 39.2% |
ANA 48.2% Anti-SSA 42.9% Anti-tRNA 35.7% |
NSIP 57.1% NSIP + OP 17.9% OP 3.6% |
CCT 81.8% MMF 76.4% AZA 36.4% |
Stable FVC and no deaths during 284.9 ± 141.3 days. |
| Ito et al. [12] |
98 | Female (58.2%) Mean age 67.5 ± 9.0 Non-smoker (54.9%) O2 usage NR |
0 | Death 27% Lung transplant NR |
– | – | ANA 47.9% RF 28.5% Anti-SSA 18.3% |
NSIP 64.3% OP 20.4% NSIP + OP 15.3% |
CCT 17.3% CCT + IS 49.0% CCT + IS + Pirfenidone 1% Pirfenidone 2% |
NSIP and age were associated with poor survival. |
(NR = non-registered; UIP = usual interstitial pneumonia; O2= Oxygen; C = clinical domain; S = serological domain; M = morphological domain; ANA = antinuclear antibody, accordingly to IPAF criteria; anti-SSA = Anti-Sjögren's-syndrome-related antigen A; RF = rheumatoid factor, accordingly to IPAF criteria; anti-tRNA = Anti-tRNA synthetase; anti-CPA = Anti-citrullinated protein antibodies NSIP = non-specific interstitial pneumonia; OP = organizing pneumonia; CCT = corticosteroids; IS = immunosuppressors; AZA = azathioprine; MMF = Mofetil mycophenolate; IPF = idiopathic pulmonary fibrosis; CTD-ILD = connective tissue disease associated interstitial lung disease; FVC = forced vital capacity).
In our cases, inflammatory arthritis was the most common clinical feature, which was different from previous studies where Raynaud's disease was described as most frequent [[8], [9], [10], [11]]. No evidence of distal digital tip ulceration, mechanics' hands or Gottron's sign was seen in our cases, probably due to high specificity of these items for specific CTDs. Considering the serology features, most patients showed an ANA titer equal to or superior to 1:320 [[8], [9], [10], [11], [12]]. Further specific antibodies proposed by the IPAF classification were not found.
Interestingly, we found an important number of features and disorders with an autoimmune “background.” (Table 2) The clinical significance of autoimmune associated conditions is unknown in IPAF; however, they can reinforce the patients’ autoimmune flavor, so we should actively look for these signs. Chartrand et al. reported that about 25% of their patients had a positive family history of CTD [11]. To our knowledge, no other study has reported other autoimmune features or diseases in IPAF patient cohorts.
NSIP is the most common HRCT pattern as stated in previous IPAF articles [[9], [10], [11], [12]]. Only two of our patients presented an UIP pattern, but Oldham et al. reported a high proportion (above 50%) of this pattern in their IPAF cohort [8]. The correct identification of the HRCT pattern is fundamental to the diagnosis, with prognostic implications in all ILDs. Some authors tried to find CT signs to differentiate CTD UIP and IPF. In their study, the straight-edge sign, anterior upper lobe sign, and exuberant honeycombing sign seemed more specific to CTD UIP [13]. The “straight edge” sign was frequently associated with the NSIP pattern, compared to UIP in another study [14]. This sign was identified in one patient (Fig. 1 – Image G); however, the clinical significance and generalized use of radiological features are still under investigation.
In our sample, most patients were treated with corticosteroids and/or an immunosuppressant with clinical and PFTs’ stability during follow-up. Nevertheless, there are no randomized controlled trials or case-control studies reporting the efficacy or safety of different immunosuppressive agents for IPAF. Data regarding treatment arises from anecdotal reports and series [6,7]. Two recent clinical trials with antifibrotics (pirfenidone for unclassifiable ILD and nintedanib for progressive fibrosing-ILD), included subjects fulfilling IPAF criteria, demonstrating a benefit in lung function decline compared to placebo, with an acceptable safety and tolerability profile [15,16].
Our cases seemed to have a “benign disease behavior,” as no difference in PFT or mortality was verified during our long follow-up. One hypothesis may be the low percentage of oxygen usage and the UIP pattern that was seen as associated with worse outcomes [8,10]. Moreover, no new clinical, serological or morphological criteria for CTD were identified; however, the time of differentiation of UCTD was considered to be within 5 years of the literature [12,17].
Studies demonstrate that IPAF criteria overlap UCTD criteria, as well as criteria that identify early onset or incomplete forms of defined CTDs. Yet, the terms must be carefully used, as disorders might be called “IPAF” inappropriately, while representing different diseases at different stages. Some authors suggested in the multidisciplinary meeting, that the IPAF classification could be useful in selecting subsets of ILD patients at risk of developing a defined CTD, and thus deserve careful clinical vigilance. Ito et al. reported that 12.2% of IPAF patients developed other characteristics and were diagnosed with a CTD during a median follow-up of 4.5 years [12]. A detailed analysis of patients with UIP pattern, fulfilling the IPAF criteria, is also recommended, so that an IPF case is not misdiagnosed or mistreated.
Recently, some suggestions have been made to refine the IPAF definition [6,7]. The authors have proposed the exclusion of items extremely specific for CTD, the inclusion in the serological domain of anti-neutrophil cytoplasmic (ANCA) and anti-Ku antibodies, the evaluation of positive nailfold videocapillaroscopy (NVC) as exclusion criterion for IPAF, the inclusion of a rheumatologist/specialist in autoimmune diseases on the multidisciplinary team, and the clarification of the multicompartment involvement section.
In conclusion, many questions remain open regarding IPAF definition and its usage in the ILD field. As a recent concept with defined criteria, it can be a helpful tool to identify patients and promote research. Cases or series descriptions of IPAF can improve the diagnostic and therapeutic approach of this heterogeneous entity. Prospective and multicenter studies with larger samples and follow-up periods could elucidate the role of the term IPAF and recognize its prognostic impact.
Data confidentiality
The authors declare having followed the protocols in use at their working center regarding patients’ data publication.
Patients consent
Obtained for all patients.
Funding sources
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
All authors report no conflict of interest.
Contributor Information
Ana Luísa Fernandes, Email: analuisa.fernandes@ulsm.min-saude.pt.
Jorge Ferreira, Email: jorge.ferreira@ulsm.min-saude.pt.
Inês Neves, Email: inesneves.porto@gmail.com.
References
- 1.Travis W.D., Costabel U., Hansell D.M. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omote N., Taniguchi H., Kondoh Y. Lung-dominant connective tissue disease: clinical, radiologic and histologic features. Chest. 2015;148:1438–1446. doi: 10.1378/chest.14-3174. [DOI] [PubMed] [Google Scholar]
- 3.Corte T.J., Copley S.J., Desai S.R. Significance of connective tissue disease features in idiopathic interstitial pneumonia. Eur. Respir. J. 2012;39:661–668. doi: 10.1183/09031936.00174910. [DOI] [PubMed] [Google Scholar]
- 4.Vij R., Noth I., Strek M.E. Autoimmune-featured interstitial lung disease; a distinct entity. Chest. 2011;140(5):1292–1299. doi: 10.1378/chest.10-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer A., Antoniou K.M., Brown K.K. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur. Respir. J. 2015;46:976–987. doi: 10.1183/13993003.00150-2015. [DOI] [PubMed] [Google Scholar]
- 6.Sambataro G., Sambataro D., Torrisi S.E. State of the art in interstitial pneumonia with autoimmune features: a systematic review on retrospective studies and suggestions for further advances. Eur. Respir. Rev. 2018;27(148) doi: 10.1183/16000617.0139-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graney B.A., Fischer A. Interstitial pneumonia with autoimmune features. Ann Am Thorac Soc. 2019;16(5):525–533. doi: 10.1513/AnnalsATS.201808-565CME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oldham J.M., Adegunsoye A., Valenzi E. Characterization of patients with interstitial pneumonia with autoimmune features. Eur. Respir. J. 2016;47:1767–1775. doi: 10.1183/13993003.01565-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad K., Barba T., Gamondes D. Interstitial pneumonia with autoimmune features: clinical, radiologic, and histological characteristics and outcome in a series of 57 patients. Respir. Med. 2017;123:56–62. doi: 10.1016/j.rmed.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Sambataro G., Sambataro D., Torrisi S.E. Clinical, serological and radiological features of a prospective cohort of Interstitial Pneumonia with Autoimmune Features (IPAF) patients. Respir. Med. 2019;150:154–160. doi: 10.1016/j.rmed.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Chartrand S., Swigris J.J., Stanchev L., Lee J.S., Brown K.K., Fischer A. Clinical features and natural history of interstitial pneumonia with autoimmune features: a single center experience. Respir. Med. 2016;119:150–154. doi: 10.1016/j.rmed.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Ito Y., Arita M., Kumagai S. Serological and morphological prognostic factors in patients with interstitial pneumonia with autoimmune features. BMC Pulm. Med. 2017;17(1):111. doi: 10.1186/s12890-017-0453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhan X., Koelsch T., Montner S.M. Differentiating usual interstitial pneumonia from nonspecific interstitial pneumonia using high-resolution computed tomography: the "Straight-edge sign". J. Thorac. Imag. 2018;33(4):266–270. doi: 10.1097/RTI.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 14.Chung J.H., Cox C.W., Montner S.M. CT features of the usual interstitial pneumonia pattern: differentiating connective tissue disease-associated interstitial lung disease from idiopathic pulmonary fibrosis. AJR Am. J. Roentgenol. 2018;210(2):307–313. doi: 10.2214/AJR.17.18384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maher T.M., Corte T.J., Fischer A., el al Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Respir Med. 2020;8(2):147–157. doi: 10.1016/S2213-2600(19)30341-8. [DOI] [PubMed] [Google Scholar]
- 16.Flaherty K.R., Wells A.U., Cottin V. Nintedanib in progressive fibrosing interstitial lung diseases. N. Engl. J. Med. 2019;381(18):1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 17.Mosca M., Tani C., Carli L., Bombardieri S., Undifferentiated C.T.D. A wide spectrum of autoimmune diseases. Best Pract. Res. Clin. Rheumatol. 2012;26(1):73–77. doi: 10.1016/j.berh.2012.01.005. [DOI] [PubMed] [Google Scholar]