Abstract
Introduction
In Japan, several sodium glucose co-transporter 2 (SGLT2) inhibitors have been used for type 1 diabetes mellitus as an adjuvant therapy to insulin therapy; however, there are no clinical reports regarding the satisfaction of its use. Therefore, we conducted a survey among patients with type 1 diabetes undergoing treatment using an SGLT2 inhibitor.
Methods
This is a single-arm open-label prospective study including 24 patients with type 1 diabetes who were to be initiated on ipragliflozin treatment between March and August 2019. All participants provided written informed consent. They completed the Diabetes Treatment Satisfaction Questionnaire (DTSQ) for the survey and 3 months of observation after the administration of an SGLT2 inhibitor (50 mg of ipragliflozin), and changes from baseline diabetes treatment satisfaction were evaluated using modified DTSQ scores (five-step evaluation) and were analyzed.
Results
The average score for each question on DTSQ significantly increased [mean (standard deviation); 0.25 (0.25) vs 0.83 (0.77), P = 0.004]. Approximately 75% of the patients perceived an improvement in glycemic control over short periods of time. Finally, 54.2% of patients were highly satisfied and would recommend the SGLT2 inhibitor treatment [0.0 (0.0) vs. 0.92 (1.32), P < 0.001]. After the administration of ipragliflozin, reductions in body weight [24.0 (2.9) vs. 23.4 (2.9) kg/m2, P = 0.002], total insulin [39.1 (12.9) vs. 34.3 (12.5) units, P = 0.013], and glycated hemoglobin [7.77 (0.97) vs. 7.40 (0.86) %, P = 0.013] were observed, without any severe side effects. Improvements in glycemic variability indexes were observed through flash glucose monitoring.
Conclusions
SGLT2 inhibitors may improve clinical treatment satisfaction by improving glycemic variability in patients with type 1 diabetes mellitus, while not inducing severe side effects with careful use.
Trial Registration
This study is registered with the University Hospital Medical Information Network Clinical Trial Registry (UMIN000040487).
Keywords: Patient satisfaction, Sodium glucose co-transporter 2 inhibitor, Type 1 DM
Key Summary Points
| Why carry out this study? |
| In Japan, several sodium glucose co-transporter 2 (SGLT2) inhibitors have been used for type 1 diabetes mellitus; however, there are no clinical reports regarding patient satisfaction related to SGLT2 inhibitor use. |
| What did the study ask? |
| Does the administration of a SGLT2 inhibitor in type 1 diabetes mellitus improve patient satisfaction? |
| What was learned from the study? |
| SGLT2 inhibitors concurrently improved clinical treatment satisfaction and glycemic variability in patients with type 1 diabetes mellitus. |
| With careful use, SGLT2 inhibitors do not induce any severe side effects. |
Digital Features
This article is published with digital features to facilitate understanding of the article. You can access the digital features on the article’s associated Figshare page. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13227980.
Introduction
Sodium glucose co-transporter 2 (SGLT2) inhibitors improve glycemic control in patients with type 1 diabetes mellitus (DM) [1, 2]. However, the use of SGLT2 inhibitors in patients with type 1 DM elevates the risk of diabetic ketoacidosis by three-fold [3]; consequently, they are not used in the USA. For the same reason, they are used only for patients with obesity in Europe. In Japan, several SGLT2 inhibitors have been used for managing type 1 DM as an adjuvant therapy to insulin therapy. [1, 2]. Patients typically do not want to use drugs. Therefore, it may be natural to expect that addition of SGLT2 inhibitors led to worse satisfaction. However, there are no clinical reports on patient satisfaction regarding treatment with SGLT2 inhibitors for type 1 DM. Therefore, we conducted a survey targeting patients with type 1 DM undergoing treatment using the SGLT2 inhibitors.
Methods
This is a single-arm, open-label prospective study conducted at Kimitsu Chuo Hospital, Japan. This study and its protocols were approved by the institutional review board of Kimitsu Chuo Hospital, according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects issued by the Ministry of Health, Labor, and Welfare in Japan. The study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments; the Ethical Guidelines for Medical and Health Research Involving Human Subjects issued by the Ministry of Health, Labor, and Welfare in Japan; the Clinical Trials Act; and other current legal regulations in Japan. Written informed consent was obtained from all subjects after full explanation of this study. The study is registered with the University Hospital Medical Information Network Clinical Trial Registry (UMIN000040487).
Because this study was conducted at a single facility, the number of subjects was limited. Therefore, the maximum number among them was set as the recruitment target. Patients with type 1 DM, glycated hemoglobin (HbA1c) of 6.5–10.0%, and a body mass index (BMI) > 18.5 kg/m2 were included. Patients with recurrent ketoacidosis, patients with significantly poor insulin injection and oral adherence, and patients who did not consent to participate in the study were excluded.
The primary outcome was the change in satisfaction scores measured using the Diabetes Treatment Satisfaction Questionnaire (DTSQ) [4]. Satisfaction was measured using five possible responses (varying from -2, very dissatisfied, to + 2, very satisfied), at two time points: before and 3 months after 50 mg of ipragliflozin administration. Changes from baseline diabetes treatment satisfaction were evaluated using the modified DTSQ scores and were analyzed statistically using the paired t-test or Wilcoxon signed-rank test. Secondary outcomes were the assessment of the safety and efficacy of the drug. To assess the safety and efficacy of the drug, data of BMI, HbA1c, and glycemic variability were collected before and 3 months after the oral administration of the SGLT2 inhibitor using flash glucose monitoring (FGM) systems (Free Style Libre; Abbott Diabetes Care, Witney, UK). The data were analyzed statistically using the paired t-test or Wilcoxon signed-rank test. The correlations of HbA1c, mean (MEAN), standard deviation (SD), mean amplitude of glycemic excursions (MAGE), time above the range (TAR; percentage of time with glucose levels > 180 mg/dl or > 10.0 mmol/l), time in range [TIR; percentage of time with glucose levels remaining between 3.9 and 10.0 mmol/l (70–180 mg/dl)], and time below the range (TBR; percentage of time with glucose levels < 70 mg/dl or < 3.9 mmol/l) were analyzed using Spearman’s rank correlation coefficient. Multivariate analysis was performed using HbA1c, MEAN, SD, MAGE, TAR, TIR, and TBR; changes in the scores of subjective improvement in both hyperglycemia and hypoglycemia were used as explanatory variables for changes in the average score for each question. Retrospective data were collected on the insulin dose, number of severe hypoglycemic incidents requiring assistance, diabetic ketoacidosis, and other side effects.
The initial insulin dose was reduced by 10–20% before ipragliflozin administration to avoid hypoglycemia; the patients performed the insulin-dose adjustment subsequent to commencing ipragliflozin administration. Patients received guidance regarding the avoidance of diabetic ketoacidosis as per the STICH protocol by the attending physicians and nurses before ipragliflozin administration [5].
Results
From March to August 2019, 24 patients with type 1 DM, who were to receive ipragliflozin treatment were recruited. All patients responded to both the baseline and final questionnaire, and 22 were FGM users. Four patients were excluded. Three patients were unable to provide consent, and one had recurrent ketoacidosis.
The results of patient satisfaction evaluated using the modified DTSQ indicate that these patients were comparatively satisfied with the baseline treatment and had to take one additional medication; the additional medication (SGLT2 inhibitor) did not improve or worsen the score for current treatment satisfaction [mean (SD); 0.88 (0.80) vs. 1.04 (0.81), P = 0.496]. However, convenience [0.21 (0.51) vs. 0.83 (1.09), P = 0.017] and flexibility [0.08 (0.28) vs. 0.42 (0.83), P = 0.083] improved. The average score for each DTSQ significantly increased [0.25 (0.25) vs. 0.83 (0.77), P = 0.004]. An improvement was perceived by 75% of the patients regarding glycemic control over short periods of time, especially hyperglycemia for 62.5% [0.0 (0.29) vs. 0.83 (1.04), P = 0.002] of patients. In contrast, they did not experience any increase in levels of hypoglycemia [0.0 (0.0) vs. 0.16 (0.95), P = 0.376]. Finally, 54.2% of patients were highly satisfied and would recommend the SGLT2 inhibitor treatment [0.0 (0.0) vs. 0.92 (1.32), P < 0.001].
After administration of ipragliflozin, reduction in body weight [24.0 (2.9) vs. 23.4 (2.9) kg/m2, P = 0.002], total insulin [39.1 (12.9) vs. 34.3 (12.5) units, P = 0.013], and HbA1c (7.77 [0.97] vs. 7.4 [0.86] %, P = 0.013) were observed (Table 1). Severe side effects did not occur. Improvements were observed in glycemic variability indexes, such as MEAN [172.3 (26.9) vs. 155.4 (29.5) mg/dl, P < 0.001], SD [73.7 (12.8) vs. 66.7 (13.3) mg/dl, P < 0.001], MAGE [175.2 (32.8) vs. 156.9 (34.4) mg/dl, P < 0.001], TIR [51.2 (10.8) vs. 58.0 (12.5) %, P = 0.010], and TAR [41.2 (14.3) vs. 32.3 (15.5) %, P = 0.007], obtained using FGM. In contrast, TBR did not significantly increase [7.6 (7.5) vs. 9.7 (9.1) %, P = 0.074], and the percentage of time with glucose level < 54 mg/dl or < 3.0 mmol/l did not change [3.4 (4.7) vs. 4.2 (5.4) %, P = 0.138].
Table 1.
Clinical characteristics of participants and scores on the DTSQ questionnaire; data are presented as mean values (standard deviation)
| N = 24 | Baseline | Post-treatment | P-value |
|---|---|---|---|
| Sex, male/female | 16/8 | ||
| CSII/MDI | 4/20 | ||
| Patients below C-peptide sensitivity | 12 | ||
| Age | 54.2 (13.4) | ||
| BMI (kg/m2) | 24.0 (2.9) | 23.4 (2.9) | 0.002 |
| HbA1c (%) | 7.77 (0.97) | 7.40 (0.86) | 0.013 |
| BG (mg/dl) | 165.4 (61.5) | 153.6 (46.9) | 0.464 |
| C-peptide (ng/ml) | 0.27 (0.40) | 0.27 (0.41) | 0.976 |
| Scores on the questionnaire | |||
| Satisfied with the current treatment | 0.88 (0.80) | 1.04 (0.81) | 0.496 |
| Convenience of treatment | 0.21 (0.51) | 0.83 (1.09) | 0.017 |
| Flexibility of treatment | 0.08 (0.28) | 0.42 (0.83) | 0.083 |
| Willingness to recommend treatment to someone else | 0.0 (0.0) | 0.92 (1.32) | 0.005 |
| Satisfied to continue the present form of treatment | 0.08 (0.28) | 0.96 (0.91) | < 0.001 |
| Average score for each question (per patient) | 0.25 (0.25) | 0.83 (0.77) | 0.004 |
| Improved frequency of hyperglycemia | 0.0 (0.29) | 0.83 (1.04) | 0.003 |
| Improved frequency of hypoglycemia | 0.0 (0.0) | 0.16 (0.96) | 0.376 |
| Glycemic variability obtained from FGM (N = 22) | |||
| Mean (mg/dl) | 172.3 (26.9) | 155.4 (29.5) | < 0.001 |
| SD (mg/dl) | 73.7 (12.8) | 66.7 (13.3) | < 0.001 |
| MAGE (mg/dl) | 175.2 (32.8) | 156.9 (34.4) | < 0.001 |
| TIR (70–180 mg/dl) (%) | 51.2 (10.8) | 58.0 (12.5) | 0.010 |
| TAR (> 180 mg/dl) (%) | 41.2 (14.3) | 32.3 (15.5) | 0.007 |
| TBR (< 70 mg/dl) (%) | 7.6 (7.5) | 9.7 (9.1) | 0.074 |
| Glucose level of < 54 mg/dl or < 3.0 mmol/l | 3.4 (4.7) | 4.2 (5.4) | 0.138 |
| Insulin | |||
| Total insulin (unit/day) | 39.1 (12.9) | 34.3 (12.5) | 0.013 |
| Bolus insulin (unit/day) | 25.4 (9.0) | 22.0 (9.1) | 0.030 |
| Basal insulin (unit/day) | 14.7 (7.0) | 13.8 (7.2) | 0.084 |
| Side effects (number [%]) | |||
| Severe hypoglycemia that needs help from others | 0 (0.0) | ||
| Diabetic ketoacidosis | 0 (0.0) | ||
| Urinary tract infections/genital mycotic infections | 0 (0.0) | ||
| Positive urinary ketone body | 1 (4.2) | ||
| Frequent urination | 5 (20.8) | ||
| Poor physical condition | 3 (12.5) | ||
SGLT2 Sodium glucose co-transporter 2, CSII continuous subcutaneous insulin infusion, MDI multiple daily injections, BMI body mass index, BG blood glucose, FGM flash glucose monitoring, TIR time in range, TAR times above range, TBR times below range, SD standard deviation, MAGE mean amplitude of glycemic excursions
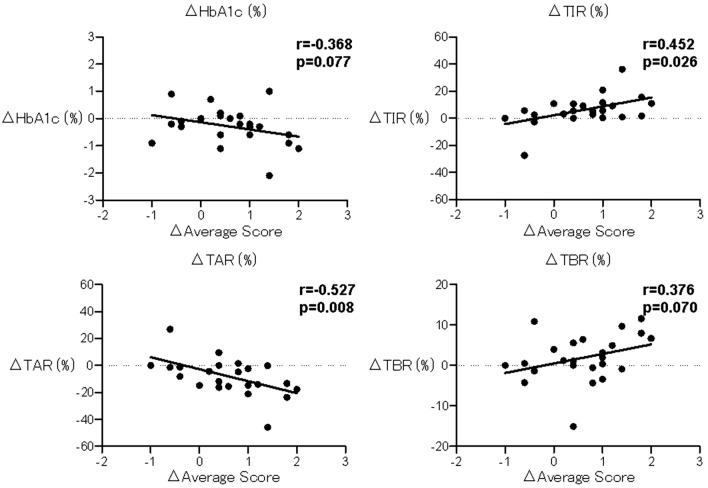
Satisfaction with the current treatment correlated with SD (r = 0.43, P = 0.035), and convenience correlated with a decrease in TAR (r = − 0.47, P = 0.043). Flexibility did not correlate with these indexes. Willingness to recommend treatment to someone else, satisfaction to continue the present form of treatment, and the average score for each question correlated with a decrease in MEAN (r = − 0.509, P = 0.011; r = − 0.551, P = 0.005, r = − 0.522, P = 0.009), a decrease in TAR (r = − 0.563, P = 0.004; r = − 0.570, P = 0.004, r = − 0.527, P = 0.008), and an increase in TIR (r = 0.493, P = 0.014; r = 0.577, P = 0.003; r = 0.452, P = 0.026) (Fig. 1). In contrast, subjective improvement in the frequency of hyperglycemia and hypoglycemia did not correlate with these indexes. Subjective improvement in the frequency of hyperglycemia correlated with improvement in convenience, flexibility, willingness to recommend treatment to someone else, satisfaction to continue the present form of treatment, and the average score for each question (r = 0.422, P = 0.031; r = 0.629, P = 0.001; r = 0.485, P = 0.016; r = 0.509, P = 0.011; r = 0.634, P = 0.001). Subjective improvement in the frequency of hypoglycemia did not correlate with changes in the score on the questionnaire. As a result of multivariate analysis, changes in the scores of subjective improvement in hyperglycemia (β = 0.510, P = 0.004) and TIR (β = 0.419, P = 0.014) were selected (R2 = 0.495, P = 0.001).
Fig. 1.
Correlations between changes in each score and blood glucose control indexes. The correlations were analyzed using Spearman’s rank correlation coefficient. TIR, time in range; TAR, times above range; TBR, times below range
Severe side effects such as severe hypoglycemia requiring help from others, diabetic ketoacidosis, or infections were not observed. Other side effects are listed in Table 1.
Discussion
Because insulin secretion is decreased in patients with type 1 DM, the amount of insulin, which is supplemented via an injection, is directly reflected in the blood, and the frequency of hypoglycemia increases when aiming for better blood glucose control. Currently, with the improvement in insulin provision and the evolution of devices such as continuous glucose monitoring/FGM and insulin pump, blood glucose levels in patients with type 1 DM can be better controlled [6–8]. Treatment with a closed-loop pump that can automatically adjust according to blood glucose levels is being implemented [9, 10]. However, it is necessary to identify further treatment methods to improve patient satisfaction.
In this study, the SGLT2 inhibitor was highly useful and satisfying for patients with type 1 DM. Data regarding the use of SGLT2 inhibitors in Europe and the US are limited because they are currently restricted owing to the high risk of developing diabetic ketoacidosis [3]. In Japan, SGLT2 inhibitors have been reported to improve glycemic control in patients with type 1 DM safely [1, 2]. Guidelines for using SGLT2 inhibitors such as the STICH protocol have been established, which may make it safer and more widely usable in the future [5]. From our data, use of an SGLT2 inhibitor for type 1 DM showed not only a decrease in MEAN but also a significant decrease in SD and MAGE. This suggested that SGLT2 inhibitors not only lower the mean of blood glucose levels but also improve the range of blood glucose fluctuation. Furthermore, it was observed that administration of SGLT2 inhibitors suppressed the increase in TBR, while decreasing TAR and increasing TIR. This result suggested that SGLT2 inhibitors act as dampers and are effective in hyperglycemia, and their pharmacologic effects lower blood glucose levels, leading to better glycemic control even in patients with type 1 DM who have a higher risk of hypoglycemia than patients with type 2 DM. In addition, as the blood glucose fluctuation range decreased, the blood glucose fluctuation patterns were simplified, making the adjustment of insulin amounts relatively easy.
Administration of SGLT2 inhibitors costs 195 yen, which is almost 1.9 dollars a day. In Japan, 30% of the burden on patients is due to health insurance; thus, the burden increased by 50 yen, which is 0.5 dollars a day, through increased administration of SGLT2 inhibitors and decreased administration of insulin. Despite the burden being increased by approximately 50 yen, which is 0.5 dollars a day, a significant improvement was observed in the average score of patient satisfaction after administration of an SGLT2 inhibitor in this study, while patient satisfaction with the original treatment was high. The patients appreciated the convenience and flexibility of this treatment. It was observed that those patients who only had the option of insulin treatment so far were highly satisfied with this treatment not only because it was an oral therapeutic drug for them but also because of its pharmacologic features. In addition, improvement in TIR and subjective hyperglycemia was found to reduce the psychologic burden on patients with hyperglycemia. The participants in this study had no serious side effects, and their glycemic control improved. Hence, the participants were willing to recommend this treatment to other patients. However, there is a limitation in this study. Because this is a small-scale, short-term, one-arm study, it does not guarantee long-term safety and efficacy. To ensure a more accurate evaluation, a long-term, large-scale study is required.
Conclusions
SGLT2 inhibitors may improve clinical treatment satisfaction and, concurrently, glycemic variability in patients with type 1 DM. With careful use, SGLT2 inhibitors also do not induce any severe side effects in a short period.
Acknowledgements
The authors thank all the participants of this study and all the clinical staff members for their assistance in the execution of the study.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical writing and Editorial Assistance
The authors thank Atsuko Watanabe and Chiho Ito for their valuable assistance with preparing the manuscript.
Prior Presentation
Part of the results of this study were disseminated at the 63rd Annual Meeting of the Japan Diabetes Society on October 5, 2020, Shiga, Japan.
Disclosures
Ryoichi Ishibashi has received research funds from Astellas Pharma Inc., Amgen Astellas BioPharma K.K., Daiichi Sankyo Co. Ltd., Taisho Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co., Ltd., MSD K.K., Boehringer Ingelheim Pharmaceuticals, Inc., and Mitsubishi Tanabe Pharma Corporation. Daigaku Uchida has received research funds from Astellas Pharma Inc., and MSD K.K. Yusuke Baba, Kyoka Kakinuma, Atsushi Takasaki, Chihiro Hiraga, Tomomi Harama, Tetsuya Yamamoto, Susumu Nakamura, Masaya Koshizaka, Yoshiro Maezawa, and Fumitaka Okajima have nothing to disclose.
Compliance With Ethics Guidelines
This study and its protocols were approved by the institutional review board of Kimitsu Chuo Hospital, according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects issued by the Ministry of Health, Labor, and Welfare in Japan. The study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments; the Ethical Guidelines for Medical and Health Research Involving Human Subjects issued by the Ministry of Health, Labor, and Welfare in Japan; the Clinical Trials Act; and other current legal regulations in Japan. Written informed consent was obtained from all subjects after full explanation of this study.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the lack of approval for data sharing from the institutional review board of Kimitsu Chuo Hospital.
References
- 1.Kaku K, Isaka H, Sakatani T, Toyoshima J. Efficacy and safety of ipragliflozin add-on therapy to insulin in Japanese patients with type 1 diabetes mellitus: a randomized, double-blind, phase 3 trial. Diabetes Obes Metab. 2019;21:2284–2293. doi: 10.1111/dom.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki D, Yamada H, Yoshida M, et al. Sodium-glucose cotransporter 2 inhibitors improved time-in-range without increasing hypoglycemia in Japanese patients with type 1 diabetes: a retrospective, single-center, pilot study. J Diabetes Investig. 2020;11:1230–1237. doi: 10.1111/jdi.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care. 2015;38:2258–2265. doi: 10.2337/dc15-1730. [DOI] [PubMed] [Google Scholar]
- 4.Bradley C. The diabetes treatment satisfaction questionnaire: DTSQ. A guide to psychological measurement in diabetes research and practice. In: Bradley C (ed). Handbook of Psychology and Diabetes. Chur: Harwood Academic Publishers; 1994:111–32.
- 5.Garg SK, Peters AL, Buse JB, Danne T. Strategy for mitigating DKA risk in patients with type 1 diabetes on adjunctive treatment with SGLT inhibitors: a STICH protocol. Diabetes Technol Ther. 2018;20:571–575. doi: 10.1089/dia.2018.0246. [DOI] [PubMed] [Google Scholar]
- 6.Jan B, Ramiro A, Petronella G, Jens K, Raimund W. Novel glucose-sensing technology and hypoglycemia in type 1 diabetes: a multicenter, non-masked, randomized controlled trial. Lancet. 2016;388:2254–2263. doi: 10.1016/S0140-6736(16)31535-5. [DOI] [PubMed] [Google Scholar]
- 7.Juvenile diabetes research foundation continuous glucose monitoring study group. effectiveness of continuous glucose monitoring in a clinical care environment. Diabetes Care 2010; 33:17–22. [DOI] [PMC free article] [PubMed]
- 8.Richard MB, William VT, Andrew A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363:311–320. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 9.Hood T, Martin T, Janet MA, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373:2129–2140. doi: 10.1056/NEJMoa1509351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lia B, Hood T, Sara H, et al. Closed-loop insulin delivery for glycemic control in noncritical care. N Engl J Med. 2018;379:547–556. doi: 10.1056/NEJMoa1805233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to the lack of approval for data sharing from the institutional review board of Kimitsu Chuo Hospital.