Abstract
The stem of Cassia siamea L. (Fabaceae) has been used in traditional Thai medicine as a longevity remedy. The objective of this study was to investigate the effect of ethanolic stem extract of C. siamea (CSE) on the life span of Drosophila melanogaster. The results showed that a diet containing 10 mg/mL CSE could significantly extend the mean life span of D. melanogaster by 14% compared with the control diet (P < 0.01). The maximum life span was 74, 78, and 84 days in control, CSE (5 mg/mL) and CSE (10 mg/mL) groups, respectively. Supplementation of CSE at 10 mg/mL also significantly increases the activity of superoxide dismutase (SOD) and catalase (CAT) at days 25 and 40 compared with the control diet. Treatment of CSE at 5 and 10 mg/mL significantly increased the climbing ability of D. melanogaster both on days 25 and 40 compared with the control flies. Paraquat and H2O2 challenge test showed that flies fed with CSE at 10 mg/mL had a longer survival time than the control flies (P < 0.01). This study provides supportive evidence that supplementation with CSE prolonged life span and reduced oxidative stress in D. melanogaster.
Keywords: Cassia siamea L., Life span, Longevity, Catalase, Superoxide dismutase, Drosophila melanogaster
Highlights
-
•
Supplementation with Cassia siamea extract extended the lifespan and improved the fly's locomotor activity
-
•
C. siamea extract supplementation increased SOD and CAT activities in the fly.
-
•
C. siamea extract supplementation improved survival after exposure to free radicals in the fly.
1. Introduction
Aging is a major risk factor for chronic diseases like cancers, cardiovascular disease, diabetes, and various degenerative diseases [1]. A remarkable challenge to the human services framework around the world is how to support and keep up a healthy life expectancy and expanding populaces of the elderly. Mechanistic studies of aging in many model organisms have led to the proposal of several hypotheses of aging. The most noticeable is the free radical theory of aging. The primary assumption of this theory is the imbalance of antioxidant defense levels and the number of oxygen species (ROS). To this respect, the improvement of the antioxidant system, as well as the decrease of ROS, should ultimately prolong the lifespan. The aging process occurs in whole systems of the body hence makes it inappropriate for in vitro studies. For aging research to be relevant to humans, Drosophila melanogaster or fruit fly is one of the most effective models for evaluating anti-aging compounds. This model organism shares a large amount of conserved biological pathways and disease-causing genes with humans [[2], [3], [4]]. Also, the fruit fly has been evidenced to be a fast and cost-effective model system in longevity research. Because of the short lifespan, the low number of chromosomes, high fecundity, and easy to handle.
There were pieces of evidence of using the extracts from plants in the increasing lifespan for over 300 years in traditional Thai medicine. Many plants are recorded as longevity medicine in ancient manuscripts, including the stem of Cassia siamea L. (Fabaceae). This plant has been used in Thai medicine as an anti-malarial infection, anti-gonorrhea, nourishing tonic, anti-fever, anti-obesity, laxative, and longevity. Previous studies have verified some of these traditional uses, such as anti-malarial infection [5], laxative [6], anti-obesity [7], antioxidant [8], and anti-inflammation [9]. Nevertheless, there are no published data on longevity property. Therefore, this study aims to investigate the longevity effect of C. siamea stem extract (CSE) in the fruit fly. To verify the mechanism of action, the antioxidant effect, and the activities of antioxidant enzymes also aimed to determine.
2. Materials and methods
2.1. Plant material and preparation of extract
C. siamea wood was collected from Plant Genetic Conservation Forest, Ubon Ratchathani Rajabhat University, Ubonratchathani Province, Thailand. Plant species were identified and authenticated by Mr. Chakkapong Thangthong, Plant Taxonomist, Ubon Ratchathani Rajabhat University, Thailand, voucher specimen number CS001. Plant materials were dried in a hot air oven at 50 °C for 48 h and ground into a fine powder. Fifty grams of powder were extracted with 250 mL ethanol using the maceration method for 7 days at ambient temperature (25–30) oC. The extracts were obtained by filtration (Whatman filter paper 40) and concentrated on a rotary evaporator (Buchi R-210, Flawil, Switzerland). The derived extracts were stored at -20 °C until use.
2.2. Culturing of Drosophila
The wild type D. melanogaster Oregon-R-C strain was obtained from the Department of Biology, Khon Kaen University. The flies were cultured in a standard wheat cream agar media seeded with yeast granules and maintained under the laboratory condition at a temperature of 25±1.2 °C of relative humidity 70–80% on a 12:12 light/dark cycle with survivors transferred to fresh food vials every 2 days. The animal study protocol was approved by the Ethic Committee of Ubon Ratchathani Rajabhat University, Thailand (Ethical Clearance No. AN63001).
2.3. The gustatory assay
The gustatory assay was performed to measure food intake. The method was modified from the previously described by Balasubramani et al. (2014) [10]. In this assay, newly eclosed male flies were reared on the control diet for seven days, then they were starved for 2 h. Sixty male flies from each experimental group were transferred into the vials containing the control diet or the extracts diets (n = 60 each, 20 per vial) containing bromophenol blue dye (0.05% wt/vol) (Sigma-Aldrich, St. Louis, MO, USA). After feeding for 2 h, the fed flies were etherized by using 5% CO2, washed with 0.1 mM phosphate-buffered saline, and homogenized in 1 mL of distilled water. Food intakes were compared by measuring the supernatant at 595 nm using a spectrophotometer (Biochrome, UK). The data were expressed as mean ± SD.
2.4. Longevity assay
Newly eclosed male flies were divided into three groups (n = 200 each, 20 per vial). The first group was reared on the standard control diet, while the other two groups were maintained on the diets containing 5 (CSE5) and 10 (CSE10) mg/mL extract, respectively. The dead flies were recorded every 2 days. The survivors were transferred to the fresh food vial containing the same diet every 2 days. Another experiment was similarly replicated, and the flies were sacrificed at days 25 and 40 in order to measure the activity of SOD and CAT, and to quantify malondialdehyde (MDA) content.
2.5. In vivo antioxidant assay
2.5.1. Intensive paraquat challenge test
Dietary paraquat (1,1′-dimethyl-4,4′-bi-pyridinium dichloride, Sigma, St. Louis, MO, USA) was used for producing superoxide anion radicals. The newly eclosed male flies were reared on the control diet or diet containing CSE5 and CSE10 (n = 200 each, 20 per vial). On day 25, all three groups of the fruit flies were starved for 2 h in an empty vial containing filter paper that soaked with distilled water and then transferred to separate vials containing a filter paper saturated with 20 mM paraquat prepared with 6% sucrose solution. Dead files were recorded every 4 h until the death of the last fly.
2.5.2. H2O2 challenge test
H2O2 was used for generating hydroxyl radical (•OH). The newly eclosed male flies were reared on the control diet or diet containing CSE5 and CSE10 (n = 200 each, 20 per vial). On day 25, all three groups of the fruit flies were starved for 2 h in an empty vial containing filter paper soaked with distilled water and then transferred to separate vials containing a filter paper saturated with 30% H2O2 prepared with 6% sucrose solution. Dead files were recorded every 4 h until the death of the last fly.
2.5.3. SOD activity
The SOD activity was measured by the indirect method using a kit provided by Sigma-Aldrich (USA) according to the supplier's protocol, adding with the preparation of samples. Briefly, the fruit flies were homogenized (n = 200, 20 flies per vial) in 1 mL of cold buffer solution (kit provided) and then centrifuged at 1500 g at 4 °C for 5 min to remove fly's debris. Off the supernatant, 900 μL were pipetted into a new microcentrifuge tube and then subjected to centrifuge at 10,000g at 4 °C for 15 min. The supernatant was used for measuring SOD1 (CuZn SOD) activity. The pellet was redissolved in 0.5 mL cold buffer and used for measuring SOD2 (Mn SOD) activity. All samples were done in triplicate.
2.5.4. CAT activity
The CAT activity was measured by the remaining hydrogen peroxide after the action of CAT present in the sample using a kit provided by Sigma-Aldrich (USA) according to the supplier's protocol. Briefly, the flies (n = 200 in 10 vials) were homogenized in 1 mL of cold enzyme dilution buffer and then centrifuged at 1500 g at 4 °C for 5 min to remove fly's debris. Off the supernatant, 500 μL were pipetted into a new microcentrifuge tube and then diluted in 20 mL of assay buffer (5 mM potassium phosphate buffer, pH 7.0). Then, 25 μL of 200 mM hydrogen peroxide solution was added to 75 μL of the diluted sample. After 1 min, 900 μL of stop solution (15 mM sodium azide) was added. Then, 10 μL of reaction mixture was mixed with 1 mL of color reagent (0.25 mM 4-aminoantipyrine, 2 mM 3,5-dichloro-2-hydroxybenzenesulfinic acid, and peroxidase (0.5–1.5 U/mg)). The reaction mixture was incubated at room temperature for 15 min and measured the absorbance at 520 nm using a spectrophotometer (Biochrome, UK). All samples were done in triplicate.
2.5.5. Measurement of lipid peroxidation
The thiobarbituric acid reaction (TBAR) method was used to determine lipid peroxidation by using malondialdehyde as a standard. The method was modified from the previously described by Devasagayam et al. (2003) [11]. The reaction mixture contained 500 μL of fly homogenate and 10 mL of TBA reagent (0.37% TBA, 15% TCA and 0.24 N HCl). The mixture was boiled for 15 min, cooled, and centrifuged to remove the precipitate (3000 rpm for 10 min). The supernatant solution's absorbance was measured at 532 nm on a spectrophotometer (Biochrome, England). The concentration of MDA in the samples was estimated using the standard 1,1,3,3- tetramethoxy propane plot.
2.6. Climbing assay
The locomotive activity of each experimental group was tested using a climbing assay as previously described with slight modification [12]. Briefly, 20 male flies were collected in each climbing assay vial and incubated for 30 min at 25 °C for adaptation. After adaptation, the flies were gently tapped down to the bottom of the test vial (2.5 X 200 mm), and given 20 s to climb up. The number of flies that reached a vertical distance of 15 cm or above was recorded. The test was performed three times on days 25 and 40.
2.7. Statistical analysis
Statistical analysis of the data was done with the SPSS 20.0 (SPSS Inc., Chicago, USA). The comparison between means was analyzed using a T-test and One-Way ANOVA. The difference between the survival curves was assessed using the Kaplan-Meier test. Differences were considered significant when P < 0.05.
3. Results
3.1. Effect of CSE on food intake of fruit flies
To ensure that the lifespan extension detected in the longevity assay was not triggered by caloric restriction, the food intake of flies was measured by using the gustatory assay. There was no significant difference observed among control and extract-fed flies (Fig. 1).
Fig. 1.
Food intake by gustatory assay of D. melanogaster in control group and diet containing 5 and 10 mg/mL CSE groups. Data are expressed as mean ± SD.
3.2. Effect of CSE on the longevity of fruit flies
Supplementation of CSE showed to extend the lifespan of fruit flies. As showed in Table 1, the 50% survival time was increased from 48 days to 58 (CSE10) and 53 (CSE5) days. The mean lifespan in control, CSE5, and CSE10 groups were 48, 53, and 58 days, respectively. The maximum lifespan was 76, 80, and 86 days in control, CSE5 and CSE10 groups, respectively. Results from the Kaplan-Meier test showed that the CSE10 treatment could significantly prolong the lifespan of fruit flies (Fig. 2). No significant difference was found in CSE5 treatment flies when compared with the control group, as well as between two extract concentration groups.
Table 1.
The 50% survival time, maximum survival of the last fly, and mean lifespan of flies fed with control diet and diets containing 5 and 10 mg/ml CSE.
| 50% survival (day) | Maximum survival (day) | Mean ± SD survival (day) | |
|---|---|---|---|
| Control | 48 | 76 | 48±1.0 |
| 5 mg/ml | 53 | 80 | 53±1.0 |
| 10 mg/ml | 58 | 86 | 57±1.0** |
**P < 0.01 when compared to control group.
Fig. 2.
Longevity effect of CSE in D. melanogaster in the control group and diet containing 5 and 10 mg/mL CSE. The statistical significance was analyzed using the Kaplan-Meier and log-rank test. The statistical significance was considered at P < 0.05.
The climbing assay was used to measure the physical performance of the flies. As shown in Fig. 3, the climbing ability decreased with age. Treatment of CSE5 and CSE10 significantly increased the climbing ability of fruit flies on days 25 (adult flies) and 40 (aged flies) compared with the control flies. However, no significant difference was observed between CSE5 and CSE10 supplemented.
Fig. 3.
The climbing ability of D. melanogaster in the control group and diet containing 5 and 10 mg/ml extract groups. Data are expressed as mean ± SD. * and ** indicate significant differences compared to the control group at P < 0.05 and P < 0.01, respectively.
3.3. Effect of CSE on the oxidative stress resistance
Free radical-induced damages are associated with aging processes. Therefore, free radical scavengers are one of the most important factors for anti-aging. As shown in Fig. 4, results on H2O2 challenge test showed that flies in the CSE10 group had a longer survival time than the control flies (P < 0.01). The maximum survival time in control, CSE5, and CSE10 groups were 16, 16 and 24 h, respectively. The 50% survival times were 7, 8, and 10 h in control, CSE5 and CSE10 groups, respectively. However, no significant difference was found between the control and 5 mg/mL CSE groups (Table 2).
Fig. 4.
Effect of CSE supplementation on the resistance to hydrogen peroxide and paraquat exposure in flies fed with control diet and diets containing 5 and 10 mg/mL CSE. The survival test was analyzed using Kaplan-Meier and log-rank test. The statistical significance was considered at P < 0.05.
Table 2.
Effect of hydrogen peroxide and paraquat treatments on the 50% survival time, maximum survival time of the last fly and mean survival time of flies fed with control and diets containing 5 and 10 mg/ml CSE.
| 50% survival (hour) | Maximum survival (hour) | Mean ± SD survival (hour) | |
|---|---|---|---|
| Hydrogen peroxide challenge test | |||
| Control | 7 | 16 | 9.31 ± 3.77 |
| 5 mg/ml CSE | 8 | 16 | 9.79 ± 3.48 |
| 10 mg/ml CSE |
10 |
24 |
12.53 ± 6.36** |
| Paraquat challenge test | |||
| Control | 14 | 24 | 16.12 ± 2.69 |
| 5 mg/ml CSE | 16 | 30 | 18.80 ± 6.22** |
| 10 mg/ml CSE | 17 | 32 | 20.07 ± 8.08** |
**P < 0.01 when compared to control group.
A similar result was investigated in the intensive paraquat challenge test. CSE 5 and CSE10 groups showed a longer survival time than in the control group. The maximum survival times in control, CSE5, and CSE10 groups were 24, 30, and 32 h, respectively. The 50% survival times were 14, 16, and 17 h in control, CSE5, and CSE10 groups, respectively. The Kaplan-Meier test showed that both CSE5 and CSE10 supplementation could significantly prolong the survival time of fruit flies (P < 0.01).
3.4. Effect of CSE on the antioxidant enzyme activities
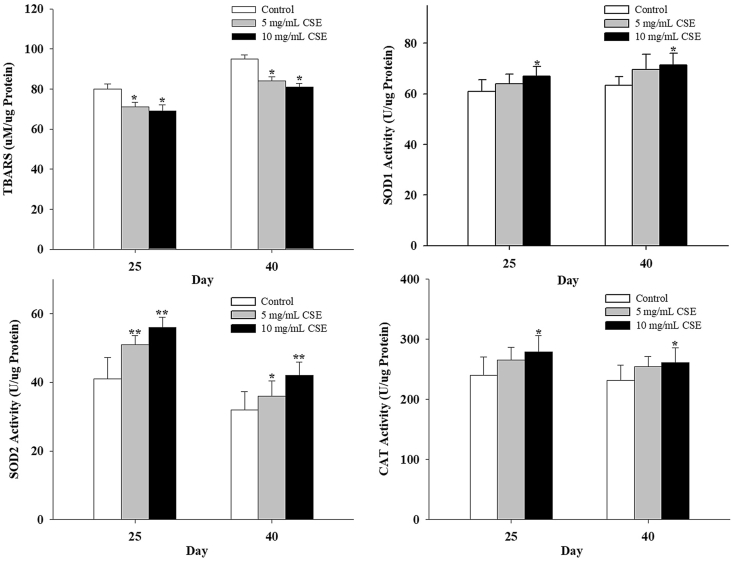
The results of the present study showed that supplementation with CSE increased SOD1, SOD2 and catalase activities in the fruit flies (Fig. 5). CSE supplementation showed to increase SOD1 and CAT activity in the flies fed with a diet containing 10 mg/mL CSE for 25 and 40 days, while supplementation with 5 mg/ml could not increase the activities of these two enzymes. As to the activity of SOD2, CSE5 and CSE10 showed to significantly increase the activity of the enzyme at days 25 and 40. The results of enzyme activities were consistent with the total body lipid peroxidation (LPO) level. 5 and 10 mg/mL CSE supplementation decreased LPO level compared with the control flies. As shown in Fig. 5, 5 and 10 mg/mL CSE supplementation for 25 and 40 days significantly decreased LPO amounts.
Fig. 5.
Effect of CSE supplementation on the MDA level and the activities of antioxidant enzymes in fruit flies reared on the control diet and diets containing 5 and 10 mg/mL CSE on the day of 25 (adult flies) and 40 (aged flies). Data are expressed as mean ± SD. *P < 0.05 compared with the value of the control group.
4. Discussion
Aging is a significant risk factor associated with the development of chronic diseases in humans, such as cancer, diabetes, cardiovascular diseases, and neurodegenerative syndromes. Many pharmacological manipulations targeting the oxidation pathway have attempted to delay aging. The present study demonstrated that supplementation of the diet containing CSE significantly improved longevity in fruit flies. We found that CSE supplementation at 10 mg/mL prolonged the mean life span from 48 to 58 days compared with the control group. The present study results could help prove that CSE could act as longevity medicine as described in the manuscript of traditional Thai medicine.
There are many theories to explain the aging process. In 1990, more than 300 theories were stated [13]. Nevertheless, the free radical theory of aging is one of the most promising theories to describe aging [14]. An imbalance between free radical generation and antioxidative capacity may cause oxidative damage to biomolecules like DNA and proteins during the aging process [15]. The Drosophila model is one of the most promising models to support this theory. The overexpression of superoxide dismutase and catalase showed to increase the maximal life span of Drosophila [16]. Also, the decreases in the activity of antioxidant enzymes were associated with age [17]. The long-lived strain of Drosophila has been shown to exhibit a more significant activity of SOD and CAT throughout its lifespan than the short-lived strain, suggesting that oxidative stress is an essential factor for aging [18]. In this study, the possible mechanism by which CSE prolongs the life span of the Drosophila may be related to its antioxidant activity. Our preliminary data showed that CSE used in the present study contained antioxidant activity with IC50 of DPPH scavenging activity at 121.25 μg/mL (data not shown). The antioxidant activity was consistent with the previous study by Mehta et al. (2017) [8], who measured C. siamea extracts' antioxidant activity and found that ethanolic extract of C. siamea contains DPPH scavenging activity with IC50 of 152.30 μg/mL. Besides its free radical scavenging activity, the life span extension action of CSE was most likely facilitated by its interaction with the activities of SOD1, SOD2, and CAT. The results of this study indicated that supplementation with CSE10 could increase the activity of SOD1, SOD2, and CAT in Drosophila. The association of anti-aging activity of CSE and the antioxidant enzyme can be verified in paraquat and H2O2 challenge tests. Our results showed that CSE supplementation could increase the survival time in Drosophila as well as decrease the level of lipid peroxidation. Our findings indicate that CSE acts as a potent antioxidant in Drosophila.
Paraquat exposure has been proved to associate with neurodegenerative disorders such as Parkinsonian syndrome. The results of this study indicated that CSE supplementation could decrease mortality and improve the locomotor activity of Drosophila. Therefore, CSE could help prevent neurodegenerative disorder.
Dietary restriction is one of the most reliable theories for retarding the aging process. Numerous experiments in various models have demonstrated that dietary restriction increases life span [[19], [20], [21]]. In this study, we examined whether the reduction of food intake was related to the life span extension effect of CSE. As evident from Fig. 1, the life span prolonging effect of CSE was not associated with calorie restriction because CSE supplementation not altered the food intake of Drosophila.
5. Conclusion
In summary, we have found that the stem extract of C. siamea, is able to increase the mean life span, decrease the paraquat and H2O2 induced mortality, and improve the locomotor activity in the Drosophila. The possible mechanism for the anti-aging property of CSE was likely associated with a protective effect against oxidative stress. The identification of the anti-aging compounds in CSE will be interested in further research.
Credit author statement
N·W performed the experiments and analyzed the data. All authors provided critical feedback and helped shape the research, analysis and manuscript. A.D. conceived and planned the experiments and wrote the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2021.100925.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.He S., Sharpless N.E. Senescence in health and disease. Cell. 2017;169(6):1000–1011. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim S.Il, Jung J.W., Ahn Y.J., Restifo L.L., Kwon H.W. Drosophila as a model system for studying lifespan and neuroprotective activities of plant-derived compounds. J. Asia Pac. Entomol. 2011;14(4):509–571. [Google Scholar]
- 3.Panchal K., Tiwari A.K. Drosophila melanogaster “a potential model organism” for identification of pharmacological properties of plants/plant-derived components. Biomed. Pharmacother. 2017;89:1331–1345. doi: 10.1016/j.biopha.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Lee S.H., Min K.J. Drosophila melanogaster as a model system in the study of pharmacological interventions in aging. Transl. Med. Aging. 2019;3:98–103. [Google Scholar]
- 5.Ajaiyeoba E.O., Ashidi J.S., Okpako L.C., Houghton P.J., Wright C.W. Antiplasmodial compounds from Cassia siamea stem bark extract. Phytother Res. 2008;22(2):254–255. doi: 10.1002/ptr.2254. [DOI] [PubMed] [Google Scholar]
- 6.Deachapunya C. Barakol extracted from Cassia siamea stimulates chloride secretion in rat colon. J. Pharmacol. Exp. Therapeut. 2005;314(2):732–737. doi: 10.1124/jpet.105.084210. [DOI] [PubMed] [Google Scholar]
- 7.Kumar D., Karmase A., Jagtap S., Shekhar R., Bhutani K.K. Pancreatic lipase inhibitory activity of cassiamin A, a bianthraquinone from Cassia siamea. Nat Prod Commun. 2013;8(2):195–198. [PubMed] [Google Scholar]
- 8.Mehta J.P., Parmar P.H., Vadia S.H., Patel M.K., Tripathi C.B. In-vitro antioxidant and in-vivo anti-inflammatory activities of aerial parts of Cassia species. Arab. J. Chem. 2017;10(2):S1654–S1662. [Google Scholar]
- 9.Nsonde Ntandou G.F., Banzouzi J.T., Mbatchi B., Elion-Itou R.D.G., Etou-Ossibi A.W., Ramos S., Benoit-Vical F., Abena A.A., Ouamba J.M. Analgesic and anti-inflammatory effects of Cassia siamea Lam. stem bark extracts. J. Ethnopharmacol. 2010;127(1):108–111. doi: 10.1016/j.jep.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 10.Balasubramani S.P., Mohan J., Chatterjee A., Patnaik E., Kukkupuni S.K., Nongthomba U., Venkatasubramanian P. Pomegranate juice enhances healthy lifespan in Drosophila melanogaster: an exploratory study. Front. Publ. Health. 2014;2:245. doi: 10.3389/fpubh.2014.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devasagayam T.P., Boloor K.K., Ramasarma T. Methods for estimating lipid peroxidation: an analysis of merits and demerits. Indian J. Biochem. Biophys. 2003;40:300–308. [PubMed] [Google Scholar]
- 12.Lee F.K.M., Wong A.K.Y., Lee Y.W., Wan O.W., Edwin Chan H.Y., Chung K.K.K. The role of ubiquitin linkages on α-synuclein induced-toxicity in a Drosophila model of Parkinson's disease. J. Neurochem. 2009;110(1):208–219. doi: 10.1111/j.1471-4159.2009.06124.x. [DOI] [PubMed] [Google Scholar]
- 13.Medvedev Z.A. An attempt at a rational classification of theories of aging. Biol. Rev. 1990;65:375–398. doi: 10.1111/j.1469-185x.1990.tb01428.x. [DOI] [PubMed] [Google Scholar]
- 14.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 15.Gutteridge J.M.C., Halliwell B. Free radicals and antioxidants in the year 2000: a historical look to the future. Ann. NY Acad. Sci. 2000;899:136–147. doi: 10.1111/j.1749-6632.2000.tb06182.x. [DOI] [PubMed] [Google Scholar]
- 16.Orr W.C., Sohal R. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 17.Sohal R.S., Arnold L., Orr W.C. Effect of age on superoxide dismutase, catalase, glutathione reductase, inorganic peroxides, TBA-reactive material, GSH/GSSG, NADPH/NADP+ and NADH/NAD+ in Drosophila melanogaster. Mech. Ageing Dev. 1990;56(3):223–235. doi: 10.1016/0047-6374(90)90084-s. [DOI] [PubMed] [Google Scholar]
- 18.Arking R., Burde V., Graves K., Hari R., Feldman E., Zeevi A., Soliman S., Saraiya A., Buck S., Vettraino J., Sathrasala K., Wehr N., Levine R.L. Forward and reverse selection for longevity in Drosophila is characterized by alteration of antioxidant gene expression and oxidative damage patterns. Exp. Gerontol. 2000;35(2):167–185. doi: 10.1016/s0531-5565(99)00094-7. [DOI] [PubMed] [Google Scholar]
- 19.McCay C.M., Crowell M.F., Maynard L.A. The effect of retarded growth upon the length of life and upon the ultimate body size. J. Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 20.Partridge L., Piper M.D., Mair W. Dietary restriction in Drosophila. Mech. Ageing Dev. 2005;126:938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Lee G.D., Wilson M.A., Zhu M., Wolkow C.A., de Cabo R., Ingram D.K., Zou S. Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell. 2006;5:515–524. doi: 10.1111/j.1474-9726.2006.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.