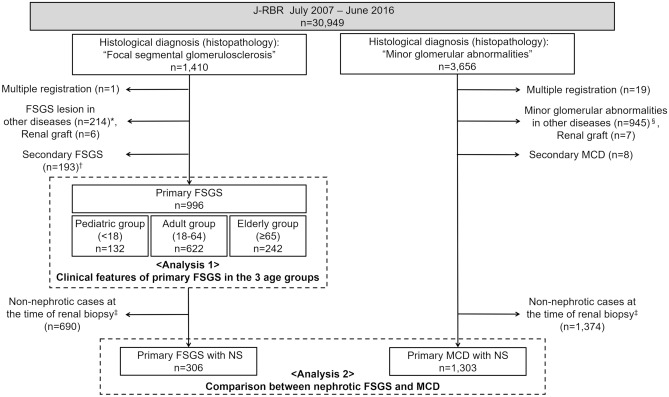
Figure 1.
Flow of patient selection in present study. *The details of distribution of histopathologically diagnosed “focal segmental glomerulosclerosis” including FSGS lesion in other diseases are shown in Supplementary Fig. S1 and Supplementary Table S1. †The details of secondary FSGS are shown in Supplementary Fig. S3. ‡Nephrotic cases at the time of renal biopsy was defined as the cases whose clinical diagnosis was nephrotic syndrome and who fitted the laboratory criteria of nephrotic syndrome. Laboratory criteria for nephrotic syndrome, for pediatric patients (age < 18): urinary protein ≥ 40 mg/h/m2 or ≥ 2.0 g/gCr and serum albumin ≤ 2.5 g/dL; for adult and elderly patients (age ≥ 18): urinary protein ≥ 3.5 g/day or ≥ 3.5 g/gCr and serum albumin ≤ 3.0 g/dL. §The details of distribution of histopathologically diagnosed “minor glomerular abnormalities” including minor glomerular abnormalities in other diseases are shown in Supplementary Fig. S2 and Supplementary Table S2. J-RBR Japan Renal Biopsy Registry, FSGS focal segmental glomerulosclerosis, MCD minimal change disease, NS nephrotic syndrome.