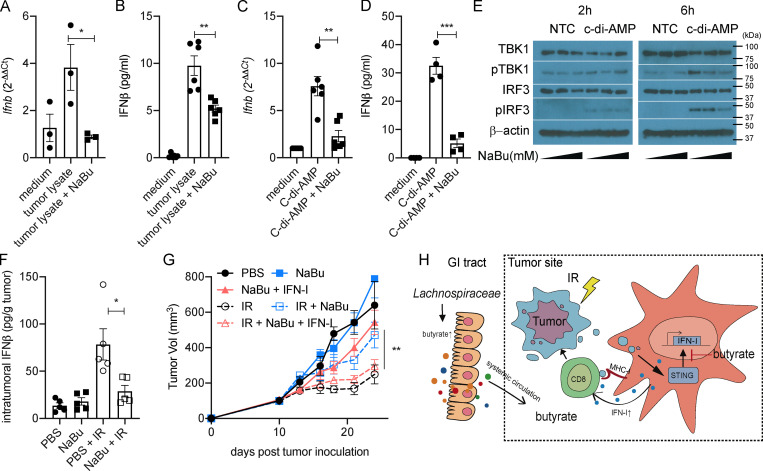
Figure 5.
Butyrate inhibits the STING activation and restrains the IR-induced intratumoral IFN-I upregulation. (A and B) BMDCs were stimulated with MC38 tumor lysate. MC38 tumor cells were irradiated with 60 Gy. 4 × 104 irradiated cells were cocultured with 106 BMDCs for 24 h. CD11c+ cells were isolated, and mRNA expression of Ifnb was detected by qPCR (A). IFNβ secretion was detected by ELISA (B). (C and D) BMDCs were stimulated with c-di-AMP (1 µg/ml) for 24 h. CD11c+ cells were isolated, and mRNA expression of Ifnb was detected by qPCR (C). IFNβ secretion was detected by ELISA (D). (E) BMDCs were stimulated with or without c-di-AMP (1 µg/ml) and NaBu (0, 0.1, or 1 mM) for 2 or 6 h. Western blot analysis of TBK1, pTBK1, IRF3, pIRF3, and β-actin is shown. (F) C57BL/6 mice (n = 5/group) were injected s.c. with 106 MC38 cells. Established tumors were irradiated (20 Gy) on day 10 after tumor inoculation. Tumor-bearing mice were intratumorally injected with 2 μmol of NaBu on day 1 after IR. Tumors were collected on day 3 after IR. IFNβ was detected by ELISA. (G) C57BL/6 mice (n = 4/group) were injected s.c. with 106 MC38 cells. Established tumors were irradiated (20 Gy) on day 10 after tumor inoculation. Tumor-bearing mice were intratumorally injected with 2 μmol of NaBu or 10 ng of recombinant IFNβ protein on days 1, 4, and 7 after IR. (H) Gut microbiota–derived butyrate suppresses radiation-induced IFN within the tumor microenvironment. Two-way ANOVA tests were used to analyze the tumor growth data. Unpaired t tests were used to analyze the other data. *, P < 0.05; **, P < 0.01; ***, P < 0.001. One representative experiment (out of two experiments in E and G or three experiments in A–D and F) is shown. GI, gastrointestinal; NTC, no-treatment control.