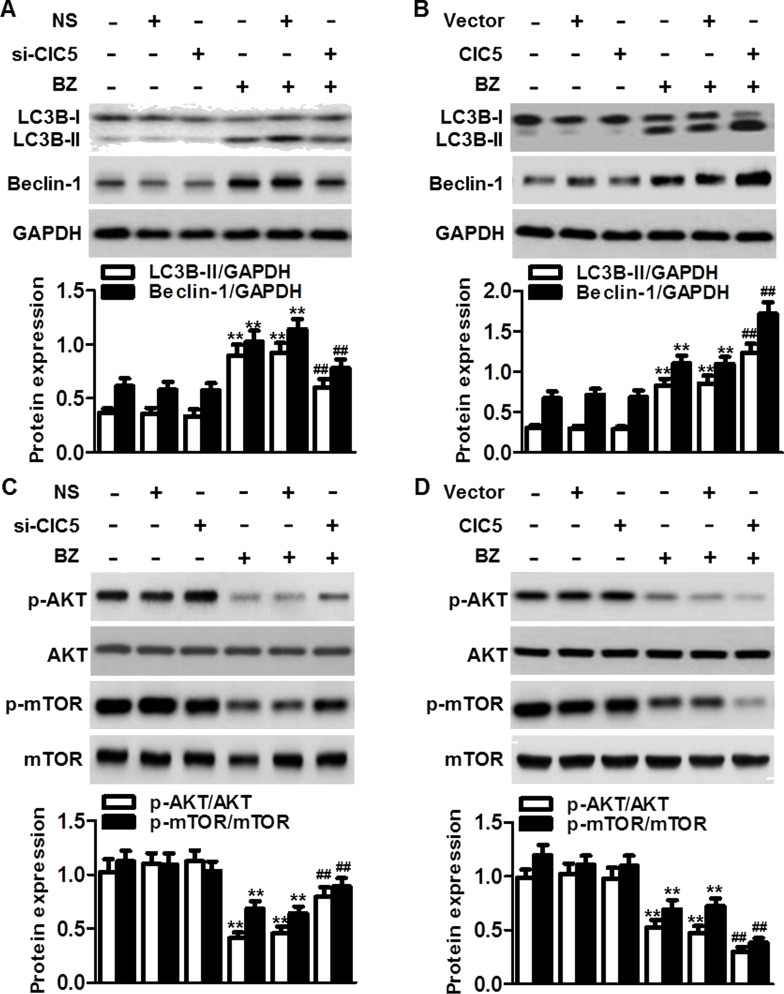
Figure 5.
ClC5 facilitated BZ-induced autophagy by inactivating the AKT–mTOR signaling pathway. ARH77 cells were transfected with ClC5 siRNA (A) or plasmid (B) for 48 h and then treated with or without BZ for another 48 h. The protein expressions of LC3B-I/II and beclin-1 were analyzed by Western blotting. GAPDH was the loading control. **p < 0.01 versus control; ##p < 0.01 versus BZ, n = 6. (C) Knockdown of ClC5 downregulation attenuated BZ-induced dephosphorylation of AKT and mechanistic target of rapamycin (mTOR). Ratios of phosphorylated to unphosphorylated AKT and mTOR, respectively, are depicted. (D) ClC5 upregulation further enhanced the inactivation of AKT–mTOR signaling induced by BZ. **p < 0.01 versus control; ##p < 0.01 versus BZ, n = 4.