Abstract
Accumulating evidence indicates that microRNA-205 (miR-205) is involved in tumor initiation, development, and metastasis in various cancers. However, its functions in neuroblastoma (NB) remain largely unclear. Here we found that miR-205 was significantly downregulated in human NB tissue samples and cell lines. miR-205 expression was lower in poorly differentiated NB tissues and those of advanced International Neuroblastoma Staging System stage. In addition, restoration of miR-205 in NB cells suppressed proliferation, migration, and invasion and induced cell apoptosis in vitro, as well as impaired tumor growth in vivo. cAMP-responsive element-binding protein 1 (CREB1) was identified as a direct target gene of miR-205. Expression of an miR-205 mimic in NB cells significantly diminished expression of CREB1 and the CREB1 targets BCL-2 and MMP9. CREB1 was also found to be upregulated in human NB tissues, its expression being inversely correlated with miR-205 expression (r = −0.554, p = 0.003). Importantly, CREB1 upregulation partially rescued the inhibitory effects of miR-205 on NB cells. These findings suggest that miR-205 may function as a tumor suppressor in NB by targeting CREB1.
Key words: Neuroblastoma (NB), miR-205, CREB1, Proliferation, Invasion
INTRODUCTION
Neuroblastoma (NB), the most common extracranial tumor among children, accounts for 7% of childhood malignancies and >15% of all cancer deaths in this age group1. Despite improvements in available treatment strategies, including surgical resection, radiotherapy, and chemotherapy, the survival rate of NB patients remains very low, since the initiation and development of this disease involve a complicated, multistep process and numerous molecular events2,3. Therefore, there is an urgent need to understand the molecular mechanisms regulating NB initiation and progression, enabling the identification of novel diagnostic markers and molecular therapeutic agents.
MicroRNAs (miRNAs) are small, endogenous, noncoding RNAs composed of approximately 19–25 nucleotides that negatively regulate gene expression by binding to the 3′-untranslated region (3′-UTR) of their target mRNAs4. It has been shown that miRNAs play crucial roles in many biological processes, including cell proliferation, differentiation, and death5,6. Increasing evidence suggests that miRNAs are involved in various aspects of tumor progression, including development, differentiation, apoptosis, proliferation, cell cycle, and metastasis, and may function as oncogenes or tumor suppressors7–9. To date, several reports have demonstrated that certain miRNAs exert substantial effects on features of NB tumorigenesis such as angiogenesis, progression, invasion, and metastasis10,11. Targeting of miRNAs may therefore have a potent therapeutic impact in this malignancy, for which they may also serve as diagnostic markers.
An increasing number of recent studies have revealed that miR-205 expression is dysregulated in various cancers and has a key function in tumor cell proliferation and migration12–18. However, given that the role of miR-205 in NB and the molecular mechanism responsible remain largely obscure, we aimed to investigate these processes in the present work.
MATERIALS AND METHODS
Patients and Tissue Samples
Twenty-eight tissue samples and matched adjacent normal tissues were collected from patients having undergone surgery at The First Hospital of Jilin University (Changchun, P.R. China) between July 2013 and July 2015. All samples were immediately frozen in liquid nitrogen following surgery and stored at −80°C until RNA extraction. Based on the Shimada index, the histology of 13 patients was classified as favorable and that of 15 as unfavorable. According to the International Neuroblastoma Staging System (INSS), 5 patients were categorized as stage 1, 8 as stage 2, 10 as stage 3, 3 as stage 4, and 2 as stage 4S. Informed consent was obtained before using samples in all cases. The study was approved by the Medical Ethics Committee of The First Hospital of Jilin University.
Cell Lines and Transfection
Four human NB cell lines, SH-SY5Y (CRL-2266), SK-N-SH (HTB-11), IMR32 (CCL-127), and BE(2)-C (CRL-2268), and human umbilical vein endothelial cells (HUVECs; CRL-1730) were purchased from the American Type Culture Collection (Manassas, VA, USA). All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA), and 100 U/ml penicillin or 100 mg/ml streptomycin at 37°C in a humidified chamber supplemented with 5% CO2.
An miR-205 mimic and a corresponding negative control (miR-NC) were purchased from GenePharma Co., Ltd. (Shanghai, P.R. China) and dissolved in diethylpyrocarbonate-treated water. The cAMP-responsive element-binding protein 1 (CREB1) overexpression plasmid (pCDNA3.1-CREB1) was provided by Dr. Jun Wang (Jilin University). Transfection was performed using Oligofectamine™ Transfection Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Quantitative Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR)
Total RNA (including miRNA) was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol and quantified with a NanoDrop ND-100 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). For measurement of miR-205 expression, qRT-PCR was performed with a TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) and a miScript SYBR Green PCR Kit (QIAGEN, Hilden, Germany) on an ABI 7900 Real-Time PCR System (Applied Biosystems). To quantify CREB1 mRNA levels, cDNA was synthesized with a PrimeScript RT Reagent Kit (Takara, Dalian, P.R. China) and then was quantified with Real-Time PCR Mixture Reagent (Takara) using specific primers, as previously described19. Relative miRNA and mRNA expression was quantified with cycle threshold (Ct) values and normalized using the 2−ΔΔCt method to U6 small nuclear RNA and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) levels, respectively.
Cell Proliferation
MTT assay was used to assess cell proliferation. Briefly, 2 × 104 transfected cells/well were seeded onto 24-well plates and cultured for 24–72 h. At each time point (24, 48, and 72 h), 100 μl of medium was replaced with an equal volume of fresh medium containing 0.5 mg/ml MTT (Sigma-Aldrich, St. Louis, MO, USA), which was incubated with cells at 37°C for 4 h. The medium was then replaced with 100 μl of dimethyl sulfoxide (DMSO; Sigma-Aldrich), and the plates were shaken at 37°C for 10 min. Absorbance at a wavelength of 570 nm was then measured in a microplate spectrophotometer (Thermo Labsystems, Vantaa, Finland). Three wells were processed in parallel for each group, and all experiments were performed in triplicate.
Cell Apoptosis Assay
Cells were stained with annexin V and 7-aminoactinomycin using an ApoScreen Annexin-V Apoptosis Kit (Southern Biotech, Birmingham, AL, USA) according to the manufacturer’s instructions. The stained cells were examined using a flow cytometer (Beckman Coulter, Brea, CA, USA) with a single 488-nm laser excitation source. Apoptosis was analyzed using CellQuest software (BD Biosciences, San Jose, CA, USA).
Cell Migration Assay
Cell migration was determined by wound healing assays. Briefly, transfected cells were cultured in six-well plates (5 × 104 cells/well) for 24 h. These cultures were then scratched using a sterile plastic micropipette tip to create an artificial wound. Photographs were taken 24 h later using an X71 inverted microscope (Olympus, Tokyo, Japan).
Cell Invasion Assay
Cell invasion was measured using Matrigel invasion assays. Briefly, 2 × 105 transfected cells in serum-free DMEM were seeded into the upper BD BioCoat Matrigel Invasion Chamber (BD Biosciences), and 750 μl of medium containing 10% FBS as a chemoattractant was added to the lower chamber. The cells were incubated for 24 h, after which noninvading cells were removed with a cotton swab. Invading cells were fixed in 20% methanol and stained with 0.1% crystal violet. Under an X71 inverted microscope (Olympus), the fixed cells in five randomly selected fields were photographed and counted.
Identification of Putative Binding Targets for miR-205
The databases TargetScan (http://www.targetscan.org) and miRanda (http://microrna.org) were used to identify putative targets for miR-205. The likely target CREB was identified, and its expression was investigated.
Luciferase Reporter Assay
Human CREB1 3′-UTR sequences containing the wild-type (Wt) or mutant (Mut) miR-205 binding region were subcloned into the psiCHECK-2 vector (Promega) between the XhoI and NotI sites. These constructs were termed CREB1-Wt-3′-UTR and CREB1-Mut-3′-UTR, respectively. Cells were seeded into 24-well plates and cultured for 24 h before being cotransfected with the CREB1-Wt-3′-UTR or CREB1-Mut-3′-UTR reporter plasmid (100 ng) and the miR-205 mimic or miR-NC (100 nM). Forty-eight hours after transfection, luciferase activity was determined using the Dual-Luciferase Reporter System (Promega).
Western Blotting
Cells or tissues were incubated on ice with lysis buffer [50 mM Tris-HCl (pH 7.5), 20 mM NaCl, 5 mM ethylenediaminetetraacetic acid (EDTA), 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), 5% glycerol, and protease inhibitors] and centrifuged at 20,000 × g at 4°C for 15 min. Supernatants were collected, and their protein concentrations were determined with a Pierce Bicinchoninic Acid Protein Assay Kit (Thermo Fisher, Waltham, MA, USA). Equal amounts of protein (30 μg) were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). After blocking for 1 h with 5% skimmed milk in Tris-buffered saline (TBS; 10 mM Tris and 150 mM NaCl), the membranes were incubated with the following primary antibodies overnight at 4°C, all of which were raised in mice and supplied by Santa Cruz Biotechnology (Santa Cruz, CA, USA): anti-CREB1 (1:1,000 dilution), anti-B-cell lymphoma 2 (BCL-2; 1:800), anti-matrix metalloproteinase9 (MMP9; 1:1,000), and anti-GAPDH (1:2,000). The membranes were subsequently washed three times with TBS and incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (1:5,000; Santa Cruz Biotechnology) for 2 h at room temperature. Immunoreactive protein bands were detected with an enhanced chemiluminescence-based FluorChem® FC2 imaging system (Alpha Innotech, San Jose, CA, USA).
Tumor Xenograft Model
Twenty 6-week-old male BALB/c nude mice weighing 18–20 g were obtained from the Experimental Animal Center of Changchun Institute for Biological Sciences (Changchun, P.R. China) and kept under specific pathogen-free conditions. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Jilin University.
Equal numbers of SH-SY5Y cells (2 × 106) stably expressing the miR-205 or miR-NC were suspended in 100 μl of serum-free DMEM and injected subcutaneously into the right posterior flanks of the mice (n = 10 each group), respectively. Tumor volume (V) was monitored and calculated according to the formula V = 0.5 × L × W 2, measuring tumor length (L) and width (W) every 7 days for 5 weeks. Mice were euthanized by cervical dislocation 35 days after injection, and tumor tissues were striped and weighed. Tumor tissues were embedded in paraffin and sectioned into 5-mm slices for Ki-67 immunohistochemistry. miR-205 and CREB1 expression was measured in tumor tissues by qRT-PCR and Western blotting, respectively.
Statistical Analysis
All data are reported as mean ± standard deviation (SD), and experiments were repeated at least three times. Statistical analysis was performed using SPSS 18.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). Differences were identified by a two-tailed Student’s t-test or one-way ANOVA. The relationship between CREB1 and miR-205 expression was tested with two-tailed Pearson’s correlation analysis. Values of p < 0.05 were considered statistically significant.
RESULTS
miR-205 Expression Was Downregulated in NB Tissue Samples and Cell Lines
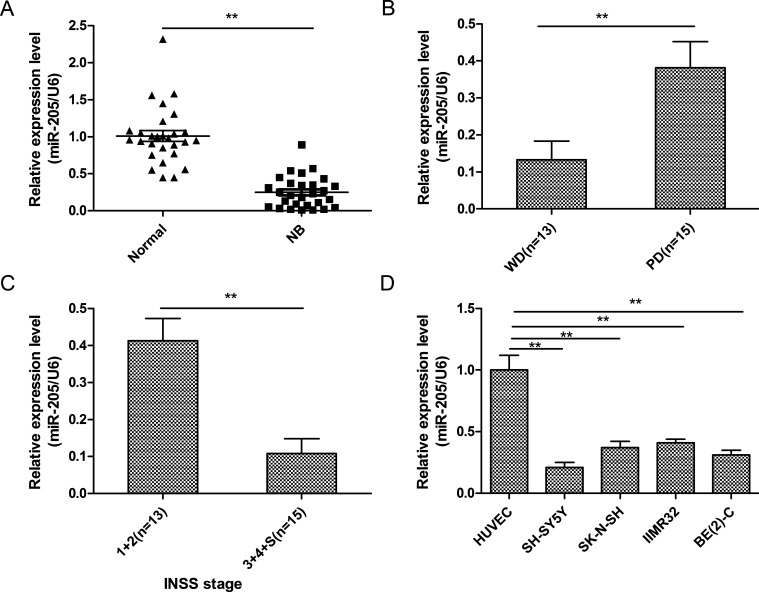
To investigate the role of miR-205 in NB occurrence, we measured its expression in specimens of NB and adjacent nontumor tissues from 28 patients using qRT-PCR. miR-205 levels were found to be decreased in NB tissues compared with adjacent normal specimens (Fig. 1A). In addition, miR-205 expression was lower in well-differentiated NB tissues (p < 0.01) (Fig. 1B) and those of advanced INSS stage (p < 0.01) (Fig. 1C). Moreover, compared to HUVECs, the four NB cell lines exhibited diminished levels of this miRNA (Fig. 1D). miR-205 expression was lowest in SH-SY5Y cells (Fig. 1D); therefore, this line was used in subsequent experiments.
Figure 1.
MicroRNA-205 (miR-205) expression was downregulated in neuroblastoma (NB) tissues and cell lines. (A) Relative miR-205 expression in 28 pairs of NB specimens and adjacent normal tissues was determined by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). (B) qRT-PCR was used to measure miR-205 levels in poorly (PD) and well-differentiated (WD) NB tissues. (C) qRT-PCR showing miR-205 levels in NB tissues of different International Neuroblastoma Staging System (INSS) stages. (D) Relative miR-205 expression in human umbilical vein endothelial cells (HUVECs) and the NB cell lines SH-SY5Y, SK-N-SH, IMR32, and BE(2)-C was determined by qRT-PCR. For all experiments in this figure, U6 small nuclear RNA (snRNA) was used as an internal control. **p < 0.01.
miR-205 Inhibited Proliferation, Migration, and Invasion and Induced Apoptosis in NB Cells
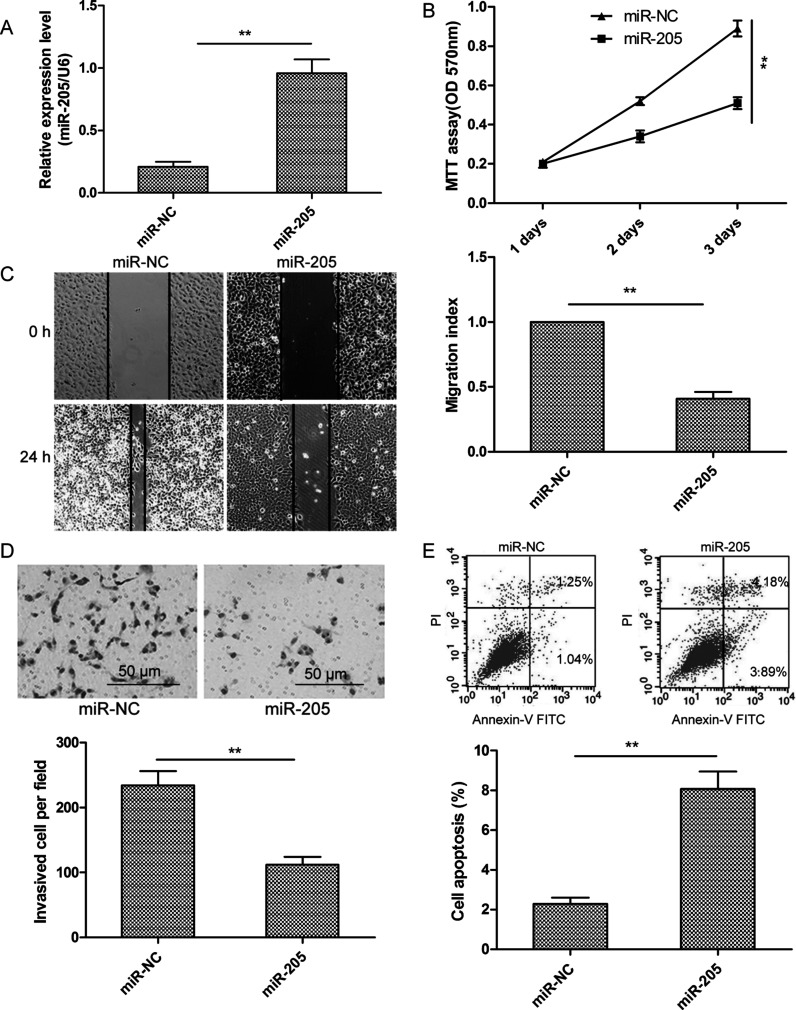
To further investigate its biological role in NB, we assessed the effects of overexpressing miR-205 in SH-SY5Y cells by transfection with an miR-205 mimic and evaluated subsequent cell proliferation, migration, invasion, and apoptosis. Transcript levels of miR-205 in SH-SY5Y cells transfected with the miR-205 mimic were shown to be increased by qRT-PCR compared to those in the miR-NC group (Fig. 2A). In comparison with the miR-NC group, expression of the miR-205 mimic significantly inhibited proliferation of SH-SY5Y cells according to the MTT assay (Fig. 2B). Wound healing and invasion assays showed that the miR-205 overexpression significantly suppressed the migration and invasion of SH-SY5Y cells (Fig. 2C and D). Flow cytometry revealed that transfection with the miR-205 mimic significantly raised the apoptosis rate of SH-SY5Y cells compared to the miR-NC group (Fig. 2E). These results suggest that miR-205 inhibits NB cell proliferation, migration, and invasion and induces cell apoptosis.
Figure 2.
Transfection with an miR-205 mimic inhibited the proliferation, migration, and invasion and induced the apoptosis of NB cells. (A) miR-205 expression level was examined by qRT-PCR after transfection of SH-SY5Y cells. U6 snRNA served as an internal control. (B–E) Cell proliferation, migration, invasion, and apoptosis were evaluated in SH-SY5Y cells transfected with the miR-205 mimic or miR-NC. **p < 0.01.
CREB1 Was Found to be a Target of miR-205 in NB Cells
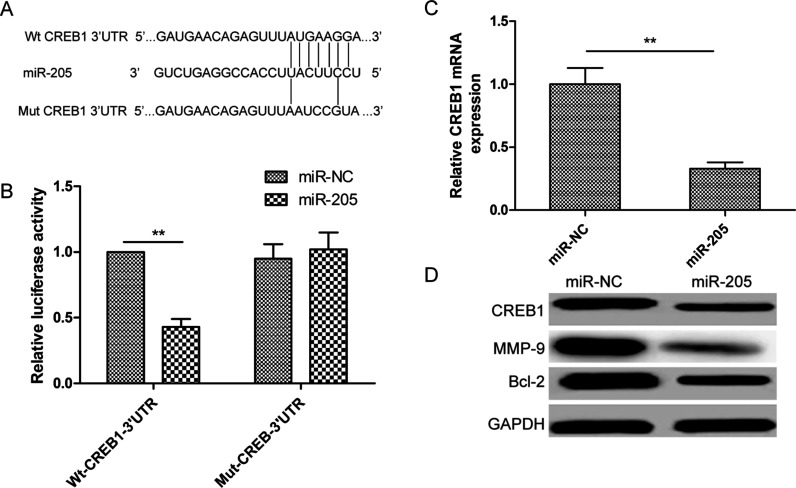
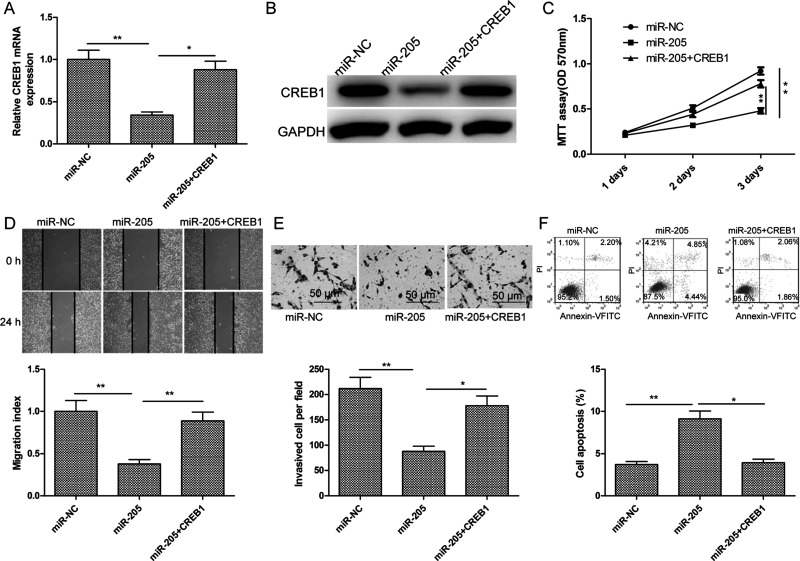
To determine the mechanism of action of miR-205 in NB cells, we performed a miRNA target search using TargetScan and miRanda. A highly conserved putative miR-205 recognition sequence was identified in the 3′-UTR of CREB1 at positions 2,036–2,043 (Fig. 3A), suggesting that this gene may be a target of miR-205. To establish whether this miRNA does target this site, we cotransfected Wt or Mut CREB1 3′-UTR constructs (Fig. 3A) and the miR-205 mimic or miR-NC into SH-SY5Ycells and carried out a luciferase assay. Expression of the miR-205 mimic in SH-SY5Y cells suppressed luciferase activity associated with the CREB1-Wt-3′-UTR plasmid, but not that resulting from the CREB1-Mut-3′-UTR plasmid (Fig. 3B). As expected, qRT-PCR and Western blotting showed that the miR-205 mimic significantly reduced CREB1 mRNA and protein expression in SH-SY5Y cells (Fig. 3C and D). Furthermore, it significantly decreased protein levels of the CREB1 targets BCL-2 and MMP9 (Fig. 3D). Thus, CREB1 may be a target of miR-205 in NB cells.
Figure 3.
cAMP-responsive element-binding protein 1 (CREB1) was found to be a target of miR-205 in NB cells. (A) Predicted miR-205 binding sites in the CREB1 mRNA sequence are shown. A mutation was introduced in the site complementary to the miR-205 seed region in the CREB1 3′-untranslated region (3′-UTR). (B) Luciferase reporter assays were performed using SH-SY5Y cells cotransfected with the miR-205 mimic or miR-NC and CREB1-Wt-3′-UTR or CREB1-Mut-3′-UTR reporter plasmid. (C) Using qRT-PCR, CREB1 mRNA levels in SH-SY5Y cells transfected with the miR-205 mimic or miR-NC were measured. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. (D) Western blotting was used to measure CREB1, B-cell lymphoma 2 (BCL-2), and matrix metalloproteinase 9 (MMP9) expression in SH-SY5Y cells transfected with the miR-205 mimic or miR-NC. GAPDH was used as a loading control. **p < 0.01.
CREB1 and miR-205 Expression Levels Were Inversely Correlated in NB Tissues
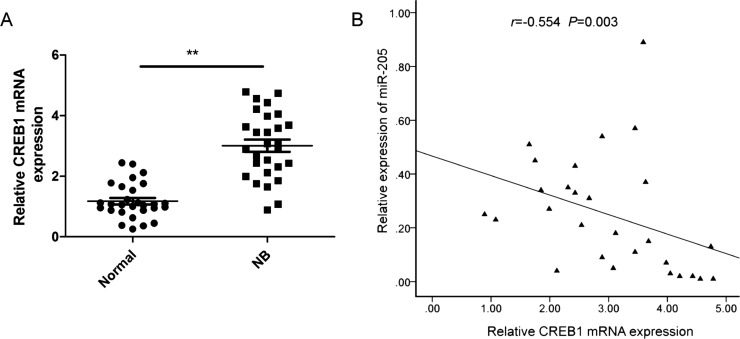
qRT-PCR revealed CREB1 mRNA expression to be greatly increased in NB specimens compared with adjacent normal tissues (Fig. 4A). In NB tissues, CREB1 transcript levels were also found to be inversely correlated with miR-205 expression by two-tailed Pearson’s correlation analysis (r = −0.643, p < 0.001) (Fig. 4B).
Figure 4.
Expression of CREB1 was inversely correlated with that of miR-205 in NB tissues. (A) CREB1 mRNA levels in 28 paired NB and adjacent normal tissue samples were determined by qRT-PCR, using GAPDH as an internal control. (B) The negative relationship between CREB1 and miR-205 expression in NB tissue samples (n = 28) was assessed by two-tailed Pearson’s correlation analysis. **p < 0.01.
CREB1 Overexpression Partially Attenuated the Effects of miR-205 in NB Cells
To determine whether the suppressive effect of miR-205 on NB cells was dependent on regulation of CREB1 expression, a CREB1 overexpression plasmid (pcDNA3.1-CREB1) was transfected into SH-SY5Y cells stably expressing the miR-205 mimic. After confirming that cotransfection with the overexpression CREB1 plasmid and miR-205 mimic obviously increased CREB1 mRNA and protein expression compared to transfection with the miR-205 mimic alone (Fig. 5A and B), the proliferation, migration, invasion, and apoptosis of the transfected cells were assayed. Restoration of CREB1 expression in SH-SY5Y cells partially reversed the effects of the miR-205 mimic on cell proliferation, migration, invasion, and apoptosis (Fig. 5C–F). These data indicate that miR-205 exerts tumor-suppressive effects on NB cells, in part by suppressing CREB1 expression.
Figure 5.
CREB1 overexpression partially attenuated the effects of miR-205 on NB cells. (A, B) CREB1 overexpression plasmid (pcDNA3.1-CREB1) was transfected into SH-SY5Y cells expressing high levels of the miR-205 mimic or miR-NC. CREB1 mRNA and protein expression was then determined by qRT-PCR and Western blotting, respectively. GAPDH was used as an internal control. (C–F) The proliferation, migration, invasion, and apoptosis of the above cells were tested by MTT, wound healing, Transwell invasion, and flow cytometry assays, respectively. *p < 0.05, **p < 0.01.
miR-205 Suppressed Tumor Growth in Nude Mice by Inhibiting CREB1
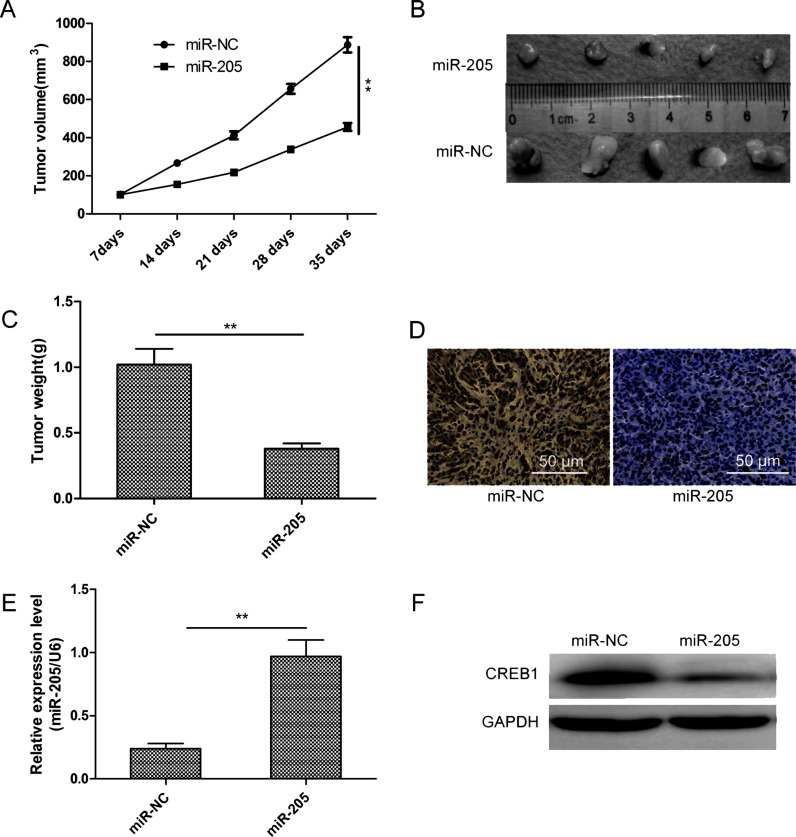
Finally, we tested whether miR-205 expression could influence the growth of NB in vivo. SH-SY5Y cells stably expressing the miR-205 mimic or miR-NC were injected subcutaneously into nude mice, and tumor sizes were measured every 7 days for 35 days. Tumor growth curves showed progressive expansion of SH-SY5Y cells transfected with miR-NC, while those transfected with the miR-205 mimic exhibited slower growth (Fig. 6A). Following euthanasia of mice and tissue collection, we found that tumor size and weight in the miR-205 mimic group were significantly smaller than those of the miR-NC group (Fig. 6B and C).
Figure 6.
miR-205 suppressed tumor growth in nude mice by inhibiting CREB1. (A) Tumor growth curves were established by measuring tumor volume every 7 days after injection. (B) Photographs of xenograft tumors isolated from nude mice in each treatment group on day 35 after injection. (C) Weight of xenograft tumors isolated from nude mice in each treatment group on day 35 after injection. (D) Ki-67 immunochemistry assays were used to assess the inhibitory effects of miR-205 in xenograft tumors. (E) Relative miR-205 expression in xenograft tumors was determined by qRT-PCR, using U6 snRNA as an internal control. (F) CREB1 protein expression in xenograft tumors was determined by Western blotting, with GAPDH serving as a loading control. **p < 0.01.
Moreover, we found that the protein level of Ki-67, a molecular biomarker for proliferation, was obviously decreased in the miR-205 mimic group compared to the miR-NC group (Fig. 6D). Finally, measurement of miR-205 and CREB1 expression by qRT-PCR and Western blotting, respectively, showed that tumors derived from miR-205 mimic-transfected cells contained higher numbers of miR-205 transcripts (Fig. 6E) and lower CREB1 protein levels (Fig. 6F). This implies that miR-205 can restrict NB tumorigenicity in vivo by suppressing CREB1 expression.
DISCUSSION
Accumulating evidence has shown that miRNAs are involved in the initiation and development of NB and may serve as effective molecular biomarkers for cancer diagnosis, prognosis, and therapy10,11. In the present study, miR-205 expression was found to be downregulated in NB cell lines and tissues and significantly negatively associated with poor differentiation and advanced INSS stage. We also demonstrated that restoration of miR-205 expression in NB cells significantly decreased their proliferation, migration, and invasion and induced their apoptosis in vitro, as well as suppressed tumor growth in vivo. These results suggest a crucial role for miR-205 in NB tumorigenesis and a possible therapeutic approach for the treatment of this disease.
miR-205, located within the second intron of the LOC642587 locus on chromosome 1, has been reported to be upregulated in liver cancer16, non-small cell lung cancer20, ovarian cancer21, and laryngeal squamous cell carcinoma13, suggesting that it functions as an oncogene in these malignancies. In contrast, several authors have reported that miR-205 is downregulated and functions as a tumor suppressor in gastric cancer17, osteosarcoma22, breast cancer12, prostate cancer23, and colorectal cancer18. These findings highlight the conflicting roles of miR-205 in different cancers, which may depend on the specific tissue and conditions in question. However, the effects of miR-205 on NB and their underlying molecular mechanisms remain unclear. Here we investigated the functions of this miR-205 in NB in vitro and in vivo, finding its expression to be significantly decreased in NB tissues and cell lines. Moreover, transfection of an miR-205 mimic suppressed NB cell growth in vitro and in vivo by targeting CREB1. Our results suggest that miR-205 might function as a tumor suppressor in this cancer.
To further investigate the molecular mechanisms underlying the influence of miR-205 on NB, we used two algorithms (TargetScan and miRanda) to identify its putative protein-coding gene targets, particularly those known to promote cancer cell proliferation, migration, and invasion. From this, CREB1 was selected, given its close association with carcinogenesis and metastasis and role as an oncogene in various cancers24,25. We subsequently generated plasmids carrying a Wt or Mut CREB1 3′-UTR sequence and cotransfected them with the miR-205 mimic or miR-NC into SH-SY5Y cells for evaluation by luciferase reporter assay, which confirmed that CREB1 was an miR-205 target. qRT-PCR and Western blotting also revealed that the expression of miR-205 mimic in NB cells significantly inhibited CREB1 expression, showing that miR-205 targets CREB1 in NB. A recent study established that miR-205 inhibits tumorigenesis and metastasis in colorectal cancer through its effect on CREB1 26. Here we extended these findings to NB occurrence and development.
CREB1, located on chromosome 2q34 in humans, encodes a transcription factor of the leucine zipper family of DNA-binding proteins27,28. Activated CREB1 can recognize the conserved cAMP-responsive element and regulate the expression of downstream genes, such as those encoding proteins involved in apoptosis (BCL-2), invasion (MMP9), cell cycle (cyclin A1, B1, and D2), signal transduction [activating transcription factor 3 and nuclear factor κ light chain enhancer of activated B cells (NF-κB)], and other growth-related genes29, associated with cell proliferation, migration, differentiation, and survival signaling pathways30. CREB1 has been shown to be a proto-oncogenic transcription factor capable of promoting tumorigenesis in many cancers, including NB31,32. In the current work, we demonstrated that CREB1 is a direct target of miR-205 in NB cells. We also found that the expression of an miR-205 mimic inhibited CREB1 and reduced levels of the downstream proteins BCL-2 and MMP9. Moreover, our data indicate that CREB1 was upregulated in NB tissues, in which expression of its mRNA negatively correlated with that of miR-205. Of note, overexpression of CREB1 partially reversed the effects of miR-205 on cell proliferation, migration, invasion, and apoptosis. These results suggest that miR-205 functions as a tumor suppressor in NB, at least in part by inhibiting CREB1.
In summary, the present investigation revealed that miR-205 appears to exhibit a tumor-suppressive function in NB, at least to a certain extent by inhibiting CREB1. Elucidation of the mechanisms by which miR-205 affects NB may contribute to our understanding of the initiation and progression of this malignancy.
ACKNOWLEDGMENT
This work was supported by the National Natural Science Foundation of China (815013221).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. [DOI] [PubMed] [Google Scholar]
- 2. Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362(23):2202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo J, Dong Q, Fang Z, Chen X, Lu H, Wang K, Yin Y, Cai X, Zhao N, Chen J, Zen K, Zhang J, Zhang CY. Identification of miRNAs that are associated with tumor metastasis in neuroblastoma. Cancer Biol Ther. 2010;9(6):446–52. [DOI] [PubMed] [Google Scholar]
- 4. Ying SY, Chang DC, Lin SL. The microRNA (miRNA): Overview of the RNA genes that modulate gene function. Mol Biotechnol. 2008;38(3):257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maroney PA, Yu Y, Nilsen TW. MicroRNAs, mRNAs, and translation. Cold Spring Harb Symp Quant Biol. 2006;71:531–5. [DOI] [PubMed] [Google Scholar]
- 6. Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010;466(7308):835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature 2005;435(7043):834–8. [DOI] [PubMed] [Google Scholar]
- 8. McManus MT. MicroRNAs and cancer. Semin Cancer Biol. 2003;13(4):253–8. [DOI] [PubMed] [Google Scholar]
- 9. Calin GA, Croce CM. MicroRNA-cancer connection: The beginning of a new tale. Cancer Res. 2006;66(15):7390–4. [DOI] [PubMed] [Google Scholar]
- 10. Stallings RL. MicroRNA involvement in the pathogenesis of neuroblastoma: Potential for microRNA mediated therapeutics. Curr Pharm Des. 2009;15(4):456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Carvalho IN, de Freitas RM, Vargas FR. Translating microRNAs into biomarkers: What is new for pediatric cancer? Med Oncol. 2016;33(5):49. [DOI] [PubMed] [Google Scholar]
- 12. Elgamal OA, Park JK, Gusev Y, Azevedo-Pouly AC, Jiang J, Roopra A, Schmittgen TD. Tumor suppressive function of mir-205 in breast cancer is linked to HMGB3 regulation. PLoS One 2013;8(10):e76402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhong G, Xiong X. miR-205 promotes proliferation and invasion of laryngeal squamous cell carcinoma by suppressing CDK2AP1 expression. Biol Res. 2015;48:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jin C, Liang R. miR-205 promotes epithelial-mesenchymal transition by targeting AKT signaling in endometrial cancer cells. J Obstet Gynaecol Res. 2015;41(10):1653–60. [DOI] [PubMed] [Google Scholar]
- 15. Zhang P, Wang L, Rodriguez-Aguayo C, Yuan Y, Debeb BG, Chen D, Sun Y, You MJ, Liu Y, Dean DC, Woodward WA, Liang H, Yang X, Lopez-Berestein G, Sood AK, Hu Y, Ang KK, Chen J, Ma L. miR-205 acts as a tumour radiosensitizer by targeting ZEB1 and Ubc13. Nat Commun. 2014;5:5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao J, Xu G, Qian YW, Li YW. Down-regulation of miR-205 promotes stemness of hepatocellular carcinoma cells by targeting PLCbeta1 and increasing CD24 expression. Neoplasma 2015;62(4):567–73. [DOI] [PubMed] [Google Scholar]
- 17. Yin WZ, Li F, Zhang L, Ren XP, Zhang N, Wen JF. Down-regulation of microRNA-205 promotes gastric cancer cell proliferation. Eur Rev Med Pharmacol Sci. 2014;18(7):1027–32. [PubMed] [Google Scholar]
- 18. Orang AV, Safaralizadeh R, Hosseinpour Feizi MA, Somi MH. Diagnostic and prognostic value of miR-205 in colorectal cancer. Asian Pac J Cancer Prev. 2014;15(9):4033–7. [DOI] [PubMed] [Google Scholar]
- 19. Oerlecke I, Bauer E, Dittmer A, Leyh B, Dittmer J. Cyclic AMP enhances TGFbeta responses of breast cancer cells by upregulating TGFbeta receptor I expression. PLoS One 2013;8(1):e54261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lei L, Huang Y, Gong W. miR-205 promotes the growth, metastasis and chemoresistance of NSCLC cells by targeting PTEN. Oncol Rep. 2013;30(6):2897–902. [DOI] [PubMed] [Google Scholar]
- 21. Niu K, Shen W, Zhang Y, Zhao Y, Lu Y. MiR-205 promotes motility of ovarian cancer cells via targeting ZEB1. Gene 2015;574(2):330–6. [DOI] [PubMed] [Google Scholar]
- 22. Wang L, Shan M, Liu Y, Yang F, Qi H, Zhou L, Qiu L, Li Y. miR-205 suppresses the proliferative and migratory capacity of human osteosarcoma Mg-63 cells by targeting VEGFA. Onco Targets Ther. 2015;8:2635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang N, Li Q, Feng NH, Cheng G, Guan ZL, Wang Y, Qin C, Yin CJ, Hua LX. miR-205 is frequently downregulated in prostate cancer and acts as a tumor suppressor by inhibiting tumor growth. Asian J Androl. 2013;15(6):735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li CF, Wu WJ, Wu WR, Liao YJ, Chen LR, Huang CN, Li CC, Li WM, Huang HY, Chen YL, Liang SS, Chow NH, Shiue YL. The cAMP responsive element binding protein 1 transactivates epithelial membrane protein 2, a potential tumor suppressor in the urinary bladder urothelial carcinoma. Oncotarget 2015;6(11):9220–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang YW, Chen X, Gao JW, Zhang H, Ma RR, Gao ZH, Gao P. High expression of cAMP-responsive element-binding protein 1 (CREB1) is associated with metastasis, tumor stage and poor outcome in gastric cancer. Oncotarget 2015;6(12):10646–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li P, Xue WJ, Feng Y, Mao QS. MicroRNA-205 functions as a tumor suppressor in colorectal cancer by targeting cAMP responsive element binding protein 1 (CREB1). Am J Transl Res. 2015;7(10):2053–9. [PMC free article] [PubMed] [Google Scholar]
- 27. Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2(8):599–609. [DOI] [PubMed] [Google Scholar]
- 28. Shaywitz AJ, Greenberg ME. CREB: A stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–61. [DOI] [PubMed] [Google Scholar]
- 29. Zhang X, Odom DT, Koo SH, Conkright MD, Canettieri G, Best J, Chen H, Jenner R, Herbolsheimer E, Jacobsen E, Kadam S, Ecker JR, Emerson B, Hogenesch JB, Unterman T, Young RA, Montminy M. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci USA 2005;102(12):4459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang YW, Chen X, Ma R, Gao P. Understanding the CREB1-miRNA feedback loop in human malignancies. Tumour Biol. 2016;37(7):8487–502. [DOI] [PubMed] [Google Scholar]
- 31. Ni Y, Zhou Y, Zhou M, Zhang L. Akt and cAMP response element binding protein mediate 17beta-estradiol regulation of glucose transporter 3 expression in human SH-SY5Y neuroblastoma cell line. Neurosci Lett. 2015;604:58–63. [DOI] [PubMed] [Google Scholar]
- 32. Zamarbide M, Etayo-Labiano I, Ricobaraza A, Martinez-Pinilla E, Aymerich MS, Luis Lanciego J, Perez-Mediavilla A, Franco R. GPR40 activation leads to CREB and ERK phosphorylation in primary cultures of neurons from the mouse CNS and in human neuroblastoma cells. Hippocampus 2014;24(7):733–9. [DOI] [PubMed] [Google Scholar]