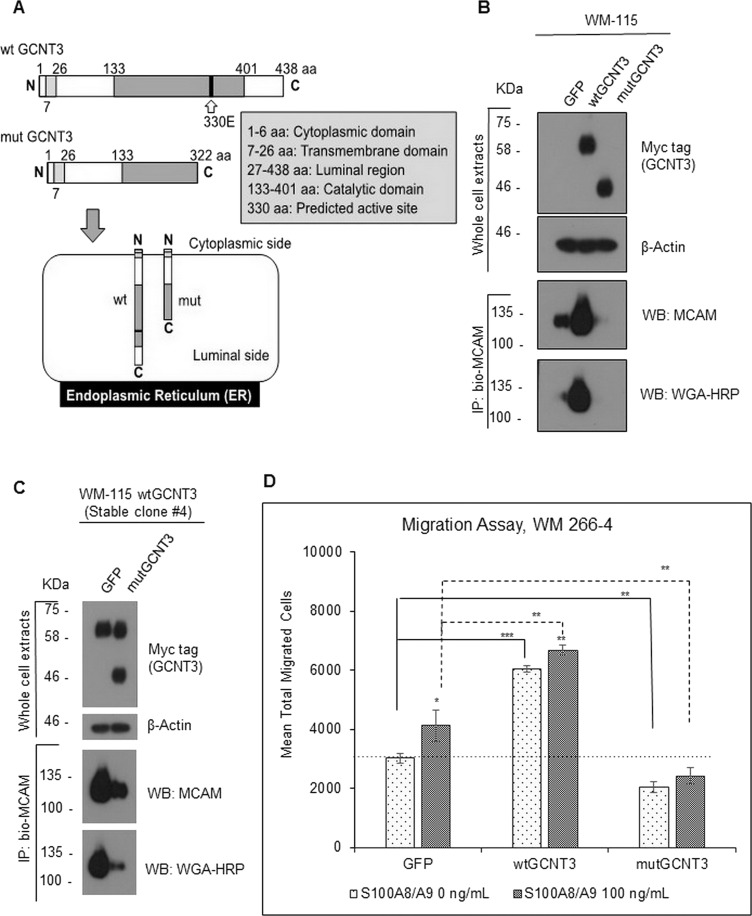
Figure 7.
Effect of catalytic dead GCNT3 (mutGCNT3) on MCAM stability and on S100A8/A9-induced migration. (A) Schematic of protein compositions of wtGCNT3 and mutGCNT3. The mutGCNT3 was designed to produce C-terminal deletion mutant of GCNT3, which lacks the catalytic domain in part but does not lose the endoplasmic reticulum (ER) localization. (B) WM-115 cells were transiently expressed with aberrant wtGCNT3, mutGCNT3, or GFP as a control for 48 h. The expressed foreign GCNT3 (wt and mut) was detected by myc antibody. After immunoprecipitation of the confirmed extracts, we used 400 mM NaCl to wash out the precipitate in the hope of removing proteins that interact with MCAM and also with the beads. The endogenous MCAM proteins and their GlcNAc levels from the prepared cell samples were determined by immunoprecipitation and following detection with either MCAM antibody or HRP-conjugated WGA. (C) The wtGCNT3-overexpressed WM-115 clone #4 with stable mode was transiently transfected with either GFP or mutGCNT3 for 48 h. The foreign expression of the GCNT3 with stable wild and temporal mutant was detected simultaneously by myc antibody, and the following immunoprecipitation and detection of the intrinsic MCAM and its GlcNAc level were carried out by a similar method as shown in (B). (D) Migration activity of WM-266-4 cells was assessed by the Boyden chamber method. The transfected cells (GFP, wtGCNT3, and mutGCNT3) in the top chamber were stimulated or not stimulated with purified recombinant S100A8/A9 (final concentration of 100 ng/ml). The quantified results are displayed. Data are shown as means ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001.