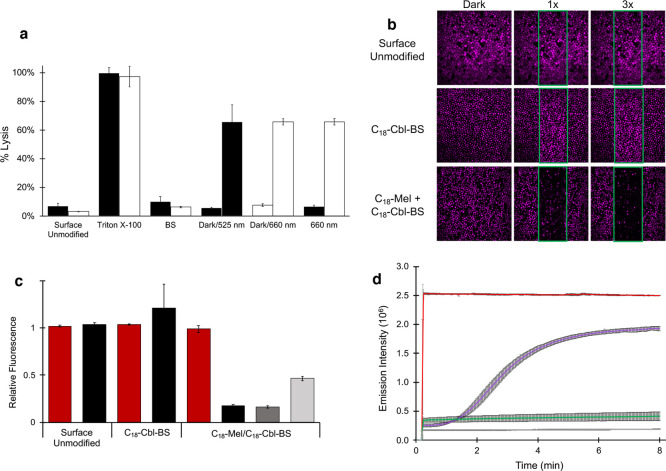
Figure 2.
a. RBC lysis as a function of various conditions. BSA-TexasRed is embedded within the interior of RBCs. Following exposure of the RBCs to various conditions, the RBCs are centrifuged, and Texas Red fluorescence in the supernatant is taken as a measure of hemolysis. Surface Unmodified RBCs: Minimal lysis is observed with RBCs lacking a surface-anchored photolytic trigger that were illuminated with 525 nm (black bar) or 660 nm (white bar) LEDs. Triton X-100-treated RBCs with surface-anchored C18-Cbl-BS/C18-Mel (black bar) or C18-Cbl-Cy5BS/C18-Mel (white bar) are used as the 100% lysis control. BS-only surface-loaded RBCs exposed to 525 nm (black bar) or 660 nm (white bar) LEDs display minimal lysis due to the absence of C18-Mel. Dark/525 nm (black bars) are RBCs containing surface-anchored C18-Cbl-BS/C18-Mel and exposed to the dark (minimal lysis) or 525 nm LEDs (66 ± 12% hemolysis relative to Triton X-100 treated RBCs). Dark/660 nm (white bars) are RBCs containing surface-anchored C18-Cbl-Cy5BS/C18-Mel and exposed to the dark (minimal lysis) or 660 nm LEDs (66 ± 2% hemolysis relative to Triton X-100 treated RBCs). 660 nm exposed RBCs containing either C18-Cbl-BS/C18-Mel (black bar) or C18-Cbl-Cy5BS/C18-Mel (white bar). Only the Cy5-containing photolytic trigger responds to 660 nm light. b. Confocal microscopy was employed to photohemolyze RBCs in a spatially resolved fashion. The RBCs were internally loaded with BSA-Alexa Fluor 647, so that they could be imaged (at 635 nm) without inducing hemolysis. Photohemolysis (region of illumination outlined in green) was performed using the onboard 515 nm laser. The images by column (left to right) are prephotolysis, a single illumination scan (10 μs/pixel), and three illumination scans. Top row: Surface Unmodified control lacks the phototrigger required for hemolysis. As expected, in the absence of the phototrigger, no RBC hemolysis is observed. Middle row: C18-Cbl-BS control lacks C18-Mel. As anticipated, in the absence of a functional phototrigger, no RBC hemolysis is observed. Bottom row: RBCs surface modified with the functional phototrigger, C18-Mel/C18-Cbl-BS, upon exposure to 515 nm, suffer hemolysis. By contrast, those RBCs outside of the illuminated area are unaffected. Scale bar represents 50 μm. c. Assessment of photohemolysis as a function of laser power. RBCs that are not surface loaded with the photolytic trigger (Surface Unmodified) do not undergo lysis as assessed by fluorescence of the RBCs in the nonilluminated (red) and illuminated (black, 515 nm, 3X 80% laser power) regions. RBCs surface modified with only C18-Cbl-BS likewise do not lyse in response to 515 illumination. By contrast, RBCs containing both C18-Cbl-BS/C18-Mel undergo robust hemolysis upon exposure to 515 nm at high (black, 3X 80% laser power), medium (dark gray, 1X 80% laser power), and low (light gray, 1X 10% laser power) illumination conditions. d. Liposomes internally loaded with fluorescently quenched 5(6)-carboxyfluorescein were surface modified with C18-Mel/C18-Cbl-BS (purple) or C18-Cbl-BS alone (gray) and subsequently exposed to 494 nm in a spectrofluorimeter to furnish both photolysis and a fluorescent readout of 5(6)-carboxyfluorescein. Controls include liposomes directly treated with C18-Mel (red) and liposomes with no surface modification (green).