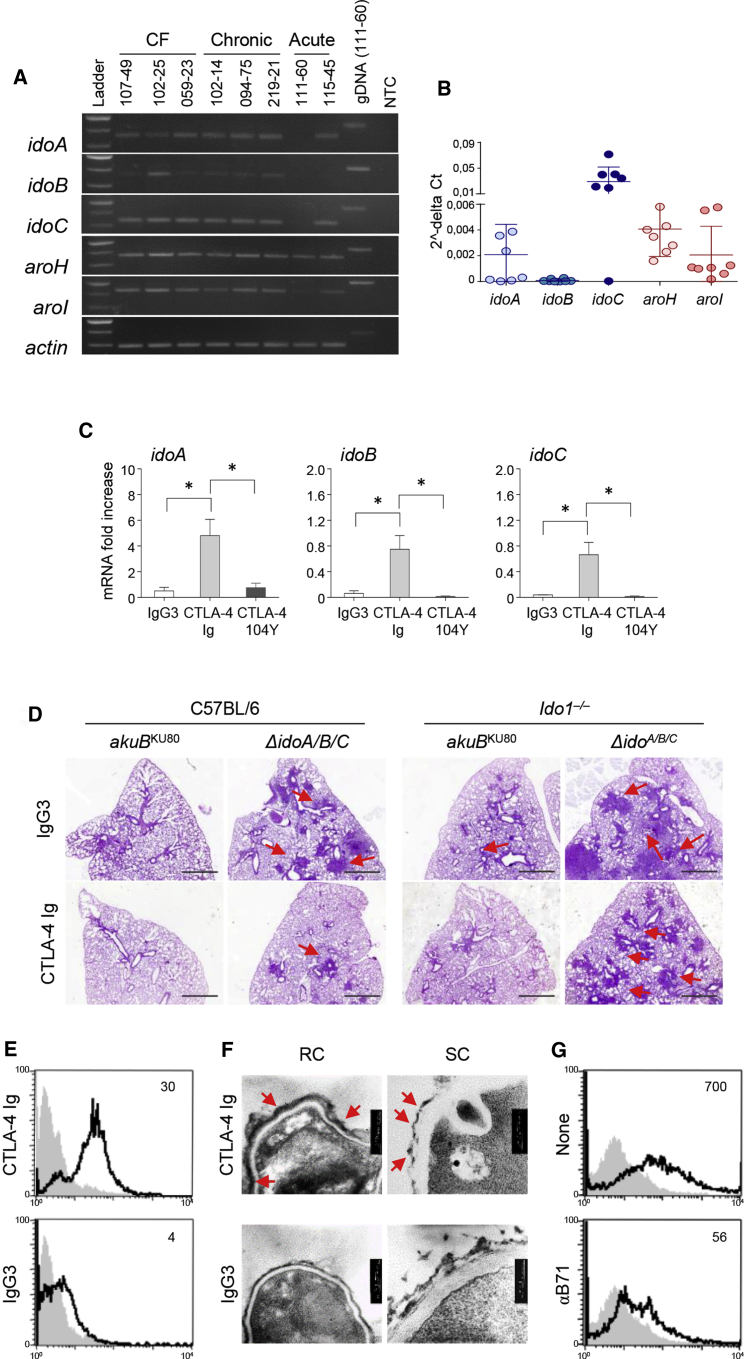
Figure 5.
Targeting fungal Idos by CTLA-4 Ig reduces Aspergillus pathogenicity
(A and B) Clinical isolates were grown overnight in liquid GMM at 37°C rotating at 200 rpm. Clinical isolates were obtained from CF patients, patients with acute invasive aspergillosis, and patients with chronic pulmonary aspergillosis. RT-PCR of Aspergillus genes involved in the catabolic pathway (Figure 2C) and (B) relative abundance (2−ΔCT) of gene expression by qPCR analysis.
(C) idoA, idoB, and idoC Aspergillus mRNA expression in Aspergillus akuBKU80 strain exposed to CTLA4-Ig, isotype control IgG3, or mutated CTLA-4 Ig (CTLA-4 104Y) overnight at 37°C.
(D) Histopathological analyses (PAS) in infected C57BL/6 and Ido1–/– mice (n = 9) upon infection with the indicated Aspergillus strains in lung tissue at 7 dpi. Mice were treated intranasally with 50 μg/mouse of CTLA-4-Ig or IgG3 for 2 days, commencing on the second day of infection. Scale bars, 10 μm.
(E) FACS analysis of Aspergillus akuBKU80 resting conidia (RC) exposed to CTLA-4-Ig or IgG3 and reacted with secondary anti-IgG3-fluorescein isothiocyanate (FITC) antibody (continuous black line). The gray histogram denotes unstained cells.
(F) Representative transmission electron microscopy (TEM) images of Aspergillus akuBKU80 RC or swollen conidia (SC) treated with CTLA-4-Ig or IgG3 followed by secondary antibodies conjugated with gold particles.
(G) Fluorescence-activated cell sorting (FACS) analysis of Aspergillus akuBKU80 RC treated with CTLA-4-Ig in the presence of anti-B71 antibody. The gray histogram denotes unstained cells.
(B and C) Data are represented as mean ± SD. Plots are representative of data collected from three independent replicate experiments. (C) Statistical significance (∗p < 0.01) was determined with one-way ANOVA and Bonferroni post hoc test. (D–G) Micrographs and FACS overlay analysis are representative of three replicate experiments.
See also Figure S8.