Figure 3.
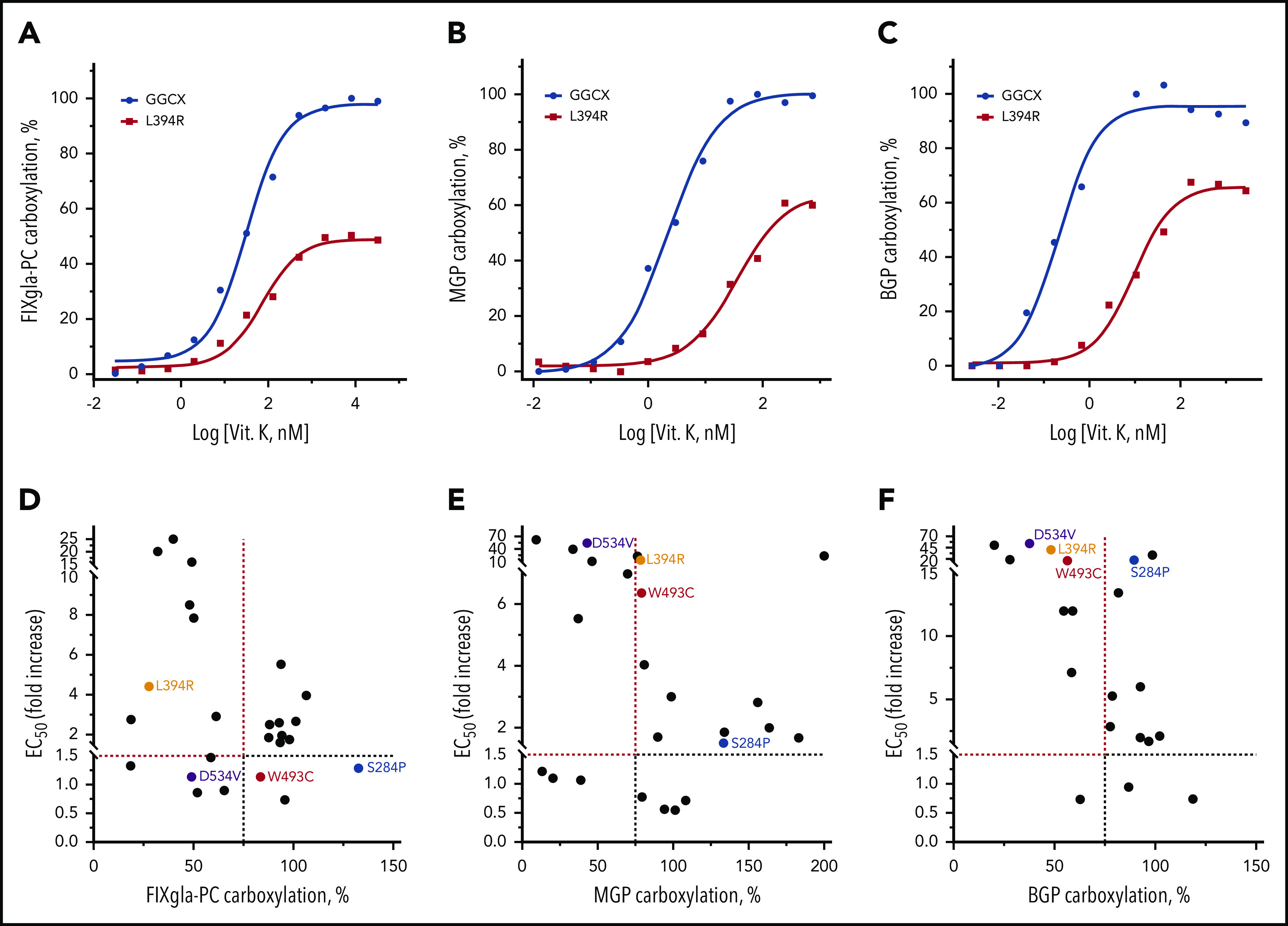
Effect of vitamin K on reporter protein carboxylation by GGCX mutations. (A-C) Vitamin K concentration titration of wild-type GGCX and the L394R mutant for the carboxylation of FIXgla-PC (A), MGP (B), and BGP (C). Wild-type GGCX or the L394R mutant was transiently expressed in GGCX-deficient HEK293 cells stably expressing FIXgla-PC, MGP, or BGP. Reporter-protein carboxylation was determined as described in Figure 1B. (D-F) Correlation between vitamin K’s EC50 and GGCX activity for the carboxylation of FIXgla-PC (D), MGP (E), and BGP (F) by GGCX mutations. The dashed lines indicate the 75% carboxylation activity (x-axis) or a 1.5-fold increase of vitamin K’s EC50. The GGCX mutations discussed in the main text were labeled with different colors.
