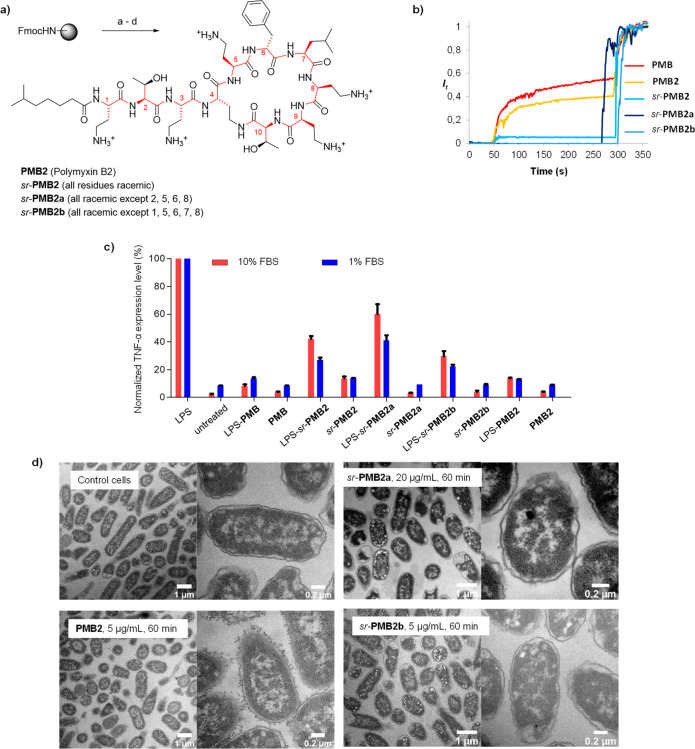
Figure 4.
Stereorandomization of polymyxin B2. (a) Solid phase total synthesis of polymyxin B2. Conditions: a) linear chain synthesis by SPPS; b) on-resin cyclization: (i) Pd(Ph3)4 (0.25 equiv), PhSiH3 (25 equiv), in dry DCM, (ii) Fmoc deprotection with 20% v/v piperidine in DMF, (iii) Oxyma (7.5 equiv), DIC (10 equiv), 25 °C,12 h ; c) TFA cleavage with TFA/TIS/H2O (94:5:1), 4 h, 25 °C, then HPLC purification. (b) Membrane-disruptive properties of commercial polymyxin and synthetic polymyxin B analogues (10 mM TRIS, 107 mM NaCl, pH 7.4). After 50 s the indicated compound was added to reach the indicated concentration. After 300 s 1.2% Triton X-100 was added for full fluorescein release. (c) Determination of TNF-α expression level in Raw264.7 murine macrophages by ELISA immunoassay. Polymyxin derivatives were coincubated with or without 0.1 μg/mL of E. coli LPS containing 1 or 10% FBS for 4 h at 37 °C and 5% CO2. (d) TEM images of E. coli cells treated for 60 min at 20 × MIC with polymyxin B analogues. see the Supporting Information for details.