Abstract
Stemless shoulder arthroplasty relies solely on cementless metaphyseal fixation and is designed to avoid stem-related problem such as intraoperative fractures, loosening, stress shielding or stress-risers for periprosthetic fractures.
Many designs are currently on the market, although only six anatomic and two reverse arthroplasty designs have results published with a minimum of two-year follow-up.
Compared to stemmed designs, clinical outcome is equally good using stemless designs in the short and medium-term follow-up, which is also the case for overall complication and revision rates.
Intraoperative fracture rate is lower in stemless compared to stemmed designs, most likely due to the absence of intramedullary preparation and of the implantation of a stem.
Radiologic abnormalities around the humeral implant are less frequent compared to stemmed implants, possibly related to the closer resemblance to native anatomy.
Between stemless implants, several significant differences were found in terms of clinical outcome, complication and revision rates, although the level of evidence is low with high study heterogeneity; therefore, firm conclusions could not be drawn.
There is a need for well-designed long-term randomized trials with sufficient power in order to assess the superiority of stemless over conventional arthroplasty, and of one design over another.
Cite this article: EFORT Open Rev 2021;6:35-49. DOI: 10.1302/2058-5241.6.200067
Keywords: complications and revisions, shoulder arthroplasty, stemless shoulder
Introduction
The latest (fourth) generation of shoulder arthroplasty includes ‘canal-sparing’ stemless designs that rely solely on metaphyseal cementless fixation, which is facilitated using either a coating which promotes ingrowth, or specific materials or configurations that promote bony ingrowth. Since its introduction in 2004, 11 different systems have been developed. These systems not only vary in the method of metaphyseal fixation with different levels of bone contact, but also in design of the taper (male or female) or collar (open, solid or absent) and the implantation technique (impaction or screw in). Stemless arthroplasty arose from the desire to avoid stem-related problems such as intraoperative fracture, loosening, stress shielding and stress risers for periprosthetic fracture.1–5 Suggested advantages are the possibility of anatomic reconstruction regardless of offset, facilitating arthroplasty in cases of proximal humeral deformity due to malunion,6 the ease of a revision,7 shorter operating time8,9 and lower amount of blood loss.9 Its popularity is confirmed by a recent market analysis which projected that the number of stemless implants will surpass conventional stemmed arthroplasty in Europe by 2024.10 Indications for stemless arthroplasty are similar to those for stemmed systems. The contraindications for stemless implants are poor metaphyseal bone stock, extensive bone cysts or avascular necrosis, proximal humeral fracture, pseudarthrosis or metabolic bone disease.
The combination of these modern humeral implants with conventional glenoid replacement implants has raised the expectation of a good functional outcome as well as long-term survivorship of stemless shoulder arthroplasty, comparable to the outcome of stemmed implants.
The primary objective of this systematic review is to summarize and analyse the clinical outcomes and the humeral-implant-related radiologic outcomes, complication and revision rates of all types of stemless shoulder arthroplasties. The secondary objective is to compare clinical outcomes between different systems.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed when conducting this study.11 The protocol was a priori registered at the International Prospective Register of Systematic Reviews (PROSPERO) (http://www.crd.york.ac.uk/prospero, registration number: CRD42020154768).
Literature search
Two reviewers (JIPW, JH) independently performed a systematic search in the online databases of Embase, Pubmed/Medline and the Cochrane library. The following search algorithm was used: “Shoulder AND (stemless OR canal sparing OR stem-free OR short stem)”. The focus of the search was on studies published in the last 10 years, from 1 January 2010 until 26 January 2020, since no study was published on this subject before 2010. We limited our search to studies written in English, Dutch or German.
The following inclusion criteria were used: Case series or comparative studies, both prospective and retrospective, (1) published in peer reviewed journals (2) reporting on outcomes of uncemented stemless hemi-, total or reverse shoulder arthroplasty (HA, TSA, RSA), (3) with a minimum follow-up of 24 months, and (4) with a minimum of five patients.
Exclusion criteria were: (1) studies reporting on short-stemmed or resurfacing arthroplasty designs, (2) abstracts without full text, (3) studies which describe similar cohorts, or (4) registry studies. When studies described similar cohorts, the study reporting the cohort with the longest follow-up was included.
After exclusion of duplicates, the titles and abstracts of the remaining articles were independently reviewed by two reviewers (JIPW, JH). Potential studies were reviewed in full text with use of the inclusion and exclusion criteria. Of the included studies, references were searched in order to identify possible additional studies that met the inclusion criteria. If there was any disagreement, consensus was reached by discussion or by consulting a third author (TDWA).
Methodological quality of studies
For each study, the level of evidence was determined using the adjusted Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence (http://www.cebm.net). Two reviewers (JIPW, JH) independently assigned the levels of evidence and assessed the methodological quality of all included studies using the Methodological Index for Non-Randomized Studies (MINORS) instrument.12 When using the non-comparative part, the highest MINORS score that can be assigned is 16, and in comparative studies the highest score is 24. If there was any disagreement, consensus was reached by discussion or by consulting a third author (TDWA).
Data extraction
All data were collected in Excel 2016 (Microsoft Corp., Redmond, WA, USA). Two reviewers extracted the data from the included articles (JIPW, JH). The following information was extracted: authors, year of publication, any conflict of interest, brand of implant, sample size, mean population age, gender, mean follow-up, glenoid type, surgical approach used, indication of surgery, patient outcomes as described below, complication and revision rates.
Outcome measures
Clinical outcome was analysed using the Constant-Murley score (CMS),13 both the absolute score and the value adjusted to age and gender,14 the American Shoulder and Elbow Surgeons score (ASES),15 Disabilities of the Arm, Shoulder and Hand score (DASH)16 and quickDASH17 score, and using the range of motion pre and post surgery. The CMS is the only outcome score that includes an objective strength and function assessment.
Additional included outcomes were radiographic outcomes, any complication as defined by the consensus report of Audigé et al,18 and any reintervention following a complication.
For the revision rate, only implant exchanges were qualified as revisions. Postoperative traumatic periprosthetic fractures were excluded from complication and revision rate comparisons, as they are not related to the implant. Radiologic changes were not included in the complication rate and mentioned separately under radiographic outcomes. A subgroup analysis was performed to compare clinical and radiologic outcomes, complication and revision rates between implant types whenever possible. An additional subgroup analysis was performed to compare CMS results between studies with different follow-up, and to compare CMS results between HA versus TSA.
Statistical analysis
All data were collected in Excel 2017 (Microsoft Corp., Redmond, WA, USA). Review Manager 5.3 (The Cochrane Collaboration, London, UK) was used to calculate differences between preoperative and postoperative outcomes and to analyse subgroup differences. Of included studies, the reported means were used or, if reported as medians, were calculated using the method described by Hozo et al.19 If standard deviations were not reported, they were calculated from the reported range using the method described by Walter et al.20 If neither a range nor a standard deviation was reported, an attempt was made to acquire these data from the corresponding author. Heterogeneity was described using the I2 test, where < 25% was considered as having no heterogeneity, low heterogeneity when < 50%, moderate when < 75% and high heterogeneity when 75% or higher.21 Considering possible high heterogeneity, a random effects model was used. All outcome data were presented as mean gain with standard deviations or 95% confidence intervals whenever possible or applicable. Differences in incidence of radiologic abnormalities, complication and revision rates were assessed using Fisher’s exact tests. All tests were two-sided and p < 0.05 was considered statistically significant.
Results
The titles and abstracts of 352 articles were screened. The literature search is summarized using the flowchart shown in Fig. 1. A total of 31 studies were included for analysis. Two randomized controlled trials (RCTs), three prospective controlled cohort studies, five retrospective controlled studies and 21 case series, of which 18 were prospective, were included (Table 1 and Table 2).
Fig. 1.
A PRISMA flow chart of the inclusion and exclusion criteria of the study.
Note. TSA, total shoulder arthroplasty; HA, hemi-arthroplasty; RSA, reverse shoulder arthroplasty.
Table 1.
Baseline characteristics of TSA/HA studies
| Study | Number of implants | Follow-up | Age | M/F | Glenoid | Approach | Indications | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors, year | Implant | Poss. COI | Total | TSA | HA | Mean, mn | Range, mn | Mean | Range | Male % | Poly/MB | DP/AS | Prim. OA | Pt. OA | ON | CTA | RA | Instab. | Other |
| Aibinder et al, 201927 | Sidus | Yes | 170 | 170 | 24.0 | 62.0 | 33–81 | 51 | Poly | DP | 100% | ||||||||
| Athwal et al, 201942 | Sidus | Yes | 86 | 86 | 24.0 | 61.0 | 33–81 | 58 | Poly | DP | 93% | 7% | |||||||
| Ballas et al, 20166 | TESS | Yes | 27 | 17 | 10 | 43.5 | 24–80 | 60.0 | 37–83 | 33 | Poly | DP* | 100% | ||||||
| Beck et al, 201828 | TESS | No | 31 | 31 | 94.7 | 76–124 | 66.7 | 34–82 | 38 | Poly/MB | DP | 65% | 10%† | 25% | |||||
| Bell and Coghlan, 201455 | Affinis | No | 12 | 12 | 24.0 | 65.9 | 49–76 | 50 | Poly | DP | 100% | ||||||||
| Bell et al, 201925 | Affinis | Yes | 23 | 23 | 24.0 | 67.5 | 49–79 | 57 | Poly | DP | 100% | ||||||||
| Berth and Pap, 20139 | TESS | No | 41 | 41 | 30.8 | 24–43 | 67.2 | 45–78 | 34 | Poly | DP | 100% | |||||||
| Brunner et al, 201230 | Eclipse | Yes | 234 | 119 | 115 | 23.2 | 2–69 | 61.5 | 32–87 | 50 | Poly/MB | DP | 44% | 31% | 3% | 1% | 7% | 13% | 2% |
| Bülhoff et al, 201931 | TESS | No | 38 | 28 | 10 | 37.1 | 24–72 | 66.4 | 55–81 | 53 | ns | AS | 100% | ||||||
| Churchill et al, 201632 | Simpliciti | Yes | 149 | 149 | 24.0 | 66.0 | 37–84 | 72 | Poly | DP | 96% | 4% | |||||||
| Collin et al, 201733 | Simpliciti | ND | 47 | 40 | 7 | 35.0 | 24–48 | 63.0 | 39–78 | 43 | ns | DP | 62% | 26% | 13% | ||||
| Gallacher et al, 201834 | Eclipse | No | 100 | 100 | 35.4 | 24–76 | 71.0 | 44–90 | 42 | ns | DP | 100% | |||||||
| Habermeyer et al, 201535 | Eclipse | Yes | 78 | 39 | 39 | 72.9 | 60–100 | 57.8 | 36–84 | 50 | Poly/MB | DP | 50% | 33% | 4% | 10% | 3% | ||
| Hawi et al, 201736 | Eclipse | Yes | 49 | 17 | 32 | 108.0 | 90–127 | 56.0 | 21–81 | 51 | Poly/MB | DP | 14% | 49% | 4% | 14% | 6% | ||
| Heuberer et al, 20188 | Eclipse | Yes | 73 | 33 | 40 | 58.1 | 48–78 | 67.6 | 40–84 | 33 | Poly | DP | 81% | 12% | 5% | 1% | |||
| Huguet et al, 201037 | TESS | Yes | 63 | 19 | 44 | 36.0 | 36–51 | 64.5 | 52–76 | 46 | Poly/MB | DP | 86%* | 14% | |||||
| Johansson et al, 201749 | Eclipse | No | 102 | 92 | 10 | 25.2 | 1–68 | 63.9 | 65 | Poly | DP | ND | |||||||
| Jordan et al, 201941 | Affinis | Yes | 35 | 24 | 11 | 24.0 | 12–67 | 65.7 | 32–88 | 8 | Poly | DP/AS | 46% | 4% | 50% | ||||
| Krukenberg et al, 201838 | Sidus | Yes | 105 | 73 | 32 | 24.0 | 64.0 | 40–79 | 50 | Poly/MB | DP | 100% | |||||||
| Moursy et al, 201940 | Eclipse | No | 23 | 2 | 21 | 90.8 | 59.9 | Poly | DP | 65% | 13% | 9% | 4% | 9% | |||||
| Razmjou et al, 201323 | TESS | No | 17 | 17 | 24.0 | 69.0 | 47 | Poly | DP | 100% | |||||||||
| Spranz et al, 201726 | TESS | No | 12 | 12 | 51.6 | 32–73 | 74.0 | 64–79 | 44 | ns | DP | 100% | |||||||
| Uschok et al, 201722 | Eclipse | Yes | 14 | 14 | 68.0 | 59–84 | 71 | Poly/MB | DP | 100% | |||||||||
| Total | 1564 | 1182 | 382 | 36.1 | 1–127 | 63.8 | 21–90 | 46% | DP 97% AS 3% | 80% | 13% | 2% | 1% | 4% | 3% | 1% | |||
Note. TSA, total shoulder arthroplasty; HA, hemi-arthroplasty; Poss. COI, possible conflict of interest; mn, month; M/F, male/female ratio; Poly, polyethylene; MB, metal backed; ns, not specified; DP, deltopectoral; AS, anterosuperior; Prim. OA, primary osteoarthritis; Pt. OA, posttraumatic osteoarthritis; ON, osteonecrosis; CTA, cuff tear arthropathy; RA, rheumatoid arthritis; Instab., Instability arthritis; ND, not described.
Posttraumatic OA and ON combined. *Primary and posttraumatic OA combined.
Table 2.
Baseline characteristics of RSA studies
| Study | Number of implants | Follow-up | Age | M/F | Approach | Indications | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors, year | Implant | Poss. COI | Total | Stemless | Short stem | Mean, mn | Range, mn | Mean | Range | Male % | DP/AS | CTA | Fract. seq. | RA | Cuff tear | Revision | Primary OA | Instab. | Hum. fract. |
| Atoun et al, 201443 | Verso | Yes | 31 | 31 | 36.0 | 24–52 | 73.5 | 58–93 | 32 | AS | 71% | 16% | 13% | ||||||
| Ballas and Béguin, 201344 | TESS | Yes | 56 | 56 | 59.0 | 38–95 | 74.0 | 55–85 | 29 | DP | 59% | 36% | 5% | ||||||
| Beck et al, 201929 | TESS | No | 29 | 12 | 17 | 101.6 | 75–142 | 72.4 | 53–88 | 19 | DP | 66% | 24% | 10% | |||||
| Kadum et al, 201424 | TESS | ND | 31 | 16 | 15 | 35.0 | 15–66 | 69.0 | 62–76 | 32 | AS | 35% | 22% | 18% | 25% | ||||
| Leonidou et al, 202045 | Verso | No | 37 | 37 | 36.0 | 12–84 | 76.9 | 25 | AS | 59% | 8% | 14% | 11% | 5% | 3% | ||||
| Levy et al, 201646 | Verso | Yes | 98 | 98 | 50.0 | 24–82 | 74.4 | 38–93 | 20 | AS | 55% | 11% | 11% | 5% | 17% | ||||
| Moroder et al, 201647 | TESS | Yes | 24 | 24 | 34.2 | DP | 100% | ||||||||||||
| Teissier et al, 201548 | TESS | Yes | 91 | 91 | 41.0 | 24–69 | 73.0 | 55–89 | 70 | DP 4 AS 87 | 100% | ||||||||
| Total | 380 | 365 | 15 | 46.1 | 12–95 | 73.6 | 38–93 | 35% | DP 23% AS 77% | 71% | 8% | 7% | 7% | 6% | 4% | 0.5% | 0.3% | ||
Note. RSA, reverse shoulder arthroplasty; Poss. COI, possible conflict of interest; mn, months; M/F, male/female ratio; DP, deltopectoral; AS, anterosuperior; CTA, cuff tear arthropathy; OA, osteoarthritis; fract. seq., fracture sequelae; RA, rheumatoid arthritis; Hum. fract., proximal humerus fracture; Instab., instability; ND, not described.
Quality assessment
According to the CEBM 2011 Levels of Evidence, there were no Level I studies. Two studies were Level II9,22 and four were Level III studies.23–26 The other 25 studies were Level IV.6,8,27–49 The results of the methodological quality assessment of studies using the MINORS criteria are summarized in Table 3. In TSA/HA literature, the average score of non-comparative studies was 10.4 out of 16 (65% of maximum, SD 2.9). The mean score in comparative studies was 14.9 out of 24 (62%, SD 1.6). In RSA literature, the average score was 10.2 (64%, SD 2.1) for non-comparative studies and 15.2 (63%, SD 0.7) for comparative studies. No blinding was applied in any of the studies. Only six studies in the TSA/HA group9,22,23,26,49 and one study in the RSA group47 compared their results to conventional stemmed arthroplasty, of which two were RCTs.9,22 In the TSA/HA group, 11 studies reported TSA results only, four presented their HA and TSA results separately and the remaining eight studies reported on the combined results of their TSA/HA cohort (Table 1).
Table 3.
Quality assessment of the included studies using the Methodological Index for Non-Randomized Studies (MINORS) criteria
| Authors | Year | Journal | Evidence | Study design | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Total | % of max. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TSA/HA studies | ||||||||||||||||||
| Aibinder et al27 | 2019 | JSES | IV | Retrospective cohort comparison | 2 | 1 | 0 | 2 | 1 | 2 | 1 | 0 | 2 | 0 | 1 | 1 | 13 | 54% |
| Athwal et al42 | 2019 | JSES | IV | Prospective case series | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 2 | 13 | 81% | ||||
| Ballas et al6 | 2016 | Intern Orthop | IV | Retrospective case series | 2 | 2 | 0 | 2 | 1 | 2 | 2 | 0 | 11 | 69% | ||||
| Beck et al28 | 2018 | Intern Orthop | IV | Prospective case series | 2 | 1 | 2 | 2 | 0 | 2 | 1 | 0 | 10 | 63% | ||||
| Bell and Coghlan55 | 2014 | IJSS | IV | Prospective case series | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 0 | 9 | 56% | ||||
| Bell et al25 | 2019 | JSES | III | Retrospective cohort comparison | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 1 | 1 | 2 | 16 | 67% |
| Berth and Pap9 | 2013 | JOT | II | Prospective randomized | 2 | 1 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 2 | 1 | 16 | 67% |
| Brunner et al30 | 2012 | Ob Extrem | IV | Prospective case series | 2 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 7 | 44% | ||||
| Bülhoff et al31 | 2019 | AOTT | IV | Retrospective case series | 2 | 1 | 0 | 2 | 0 | 2 | 1 | 0 | 8 | 50% | ||||
| Churchill et al32 | 2016 | JBJS | IV | Prospective case series | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 14 | 88% | ||||
| Collin et al33 | 2017 | Intern Orthop | IV | Prospective case series | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 11 | 69% | ||||
| Gallacher et al34 | 2018 | JSES | IV | Retrospective case series | 1 | 2 | 0 | 2 | 0 | 2 | 1 | 0 | 8 | 50% | ||||
| Habermeyer et al35 | 2015 | JSES | IV | Prospective case series | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 11 | 69% | ||||
| Hawi et al36 | 2017 | JSES | IV | Prospective case series | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 11 | 69% | ||||
| Heuberer et al8 | 2018 | BMC Musc Dis | IV | Prospective case series | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 2 | 13 | 81% | ||||
| Huguet et al37 | 2010 | JSES | IV | Prospective case series | 2 | 1 | 2 | 2 | 0 | 2 | 1 | 0 | 10 | 63% | ||||
| Johansson et al49 | 2017 | BMC Musc Dis | IV | Retrospective cohort comparison | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 1 | 2 | 1 | 2 | 14 | 58% |
| Jordan et al41 | 2019 | Musc Surg | IV | Retrospective cohort comparison | 2 | 2 | 0 | 2 | 0 | 2 | 1 | 0 | 1 | 1 | 0 | 2 | 13 | 54% |
| Krukenburg et al | 2018 | JSES | IV | Prospective case series | 2 | 2 | 2 | 2 | 0 | 1 | 1 | 2 | 12 | 75% | ||||
| Moursy et al40 | 2019 | BMC Musc Dis | IV | Prospective case series | 1 | 2 | 0 | 2 | 0 | 2 | 1 | 0 | 8 | 50% | ||||
| Razmjou et al23 | 2013 | JSES | III | Prospective controlled | 2 | 2 | 2 | 2 | 0 | 1 | 1 | 0 | 2 | 1 | 1 | 2 | 16 | 67% |
| Spranz et al26 | 2017 | BMC Musc Dis | III | Prospective controlled | 2 | 1 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 14 | 58% |
| Uschok et al22 | 2017 | JSES | II | Prospective randomized | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 2 | 2 | 1 | 1 | 17 | 71% |
| RSA Studies | ||||||||||||||||||
| Atoun et al43 | 2014 | Intern Orthop | IV | Prospective case series | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 0 | 12 | 75% | ||||
| Ballas and Béguin44 | 2013 | JSES | IV | Prospective case series | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 0 | 11 | 69% | ||||
| Beck et al29 | 2019 | AOTS | IV | Prospective case series | 1 | 1 | 2 | 2 | 0 | 2 | 1 | 0 | 9 | 56% | ||||
| Kadum et al24 | 2014 | Intern Orthop | III | Prospective controlled | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 17 | 71% |
| Leonidou et al45 | 2020 | EJOST | IV | Prospective case series | 2 | 0 | 2 | 2 | 1 | 1 | 0 | 0 | 8 | 50% | ||||
| Levy et al46 | 2016 | JSES | IV | Prospective case series | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 13 | 81% | ||||
| Moroder et al47 | 2016 | Intern Orthop | IV | Retrospective cohort comparison | 2 | 2 | 0 | 2 | 2 | 1 | 1 | 0 | 2 | 1 | 2 | 1 | 16 | 67% |
| Teissier et al48 | 2015 | JSES | IV | Prospective case series | 0 | 2 | 2 | 2 | 0 | 1 | 1 | 0 | 8 | 50% | ||||
Notes. The criteria of MINORS with 0 points when not reported, 1 when reported but not adequate, and 2 when reported and adequate. Maximum score is 16 in non-comparative studies and 24 in comparative studies.
% of max., percentage of maximum score; JSES, Journal of Shoulder and Elbow Surgery; Intern Orthop, International Orthopedics(SICOT), IJSS, International Journal of Shoulder Surgery; JOT, Journal of Orthopaedics and Traumatology; Ob Extrem, Obere Extremität; AOTT, Acta Orthopaedica et Traumatologica Turcica; JBJS, Journal of Bone and Joint Surgery; BMC Musc Dis, BioMed Central Musculoskeletal Disorders; EJOST, European Journal of Orthopaedic Surgery & Traumatology; WJO, World Journal of Orthopedics; Musc Surg, Musceloskeletal Surgery; AOTS, Archives of Orthopaedic and Trauma Surgery.
Baseline characteristics
A total of 1,903 patients with 1,944 stemless shoulder prostheses were included in this study, of which 1,182 were anatomic total shoulder arthroplasties, 365 were reverse shoulder arthroplasties and 382 were hemi shoulder arthroplasties. The average age in the TSA/HA group was 64 years (range 21–90) and in the RSA group 73 years (range 38–93), overall mean follow-up was 3.2 years, and 55% of patients were female (Tables 1 and 2).
Of the included TSA/HA studies, the results of the TESS (Biomet, Warsaw, IN, USA) and Eclipse (Arthrex, Freiham, Germany) implants are described most frequently. Other included studies described the Affinis Short (Mathys AG, Bettlach, Switzerland), Sidus Stem-Free (Zimmer Biomet, Warsaw, IN, USA) and Simpliciti (Wright Medical, Memphis, TN, USA). In RSA literature, only the results of the TESS RSA system and Verso system (Innovative Design Orthopaedics, London, UK) have been published. A medium-term follow-up (60–120 months) was described in five TSA/HA articles and one RSA article. Eighteen TSA/HA and seven RSA studies described a short-term follow-up (24–60 months). To our knowledge, no long-term data have been published on any stemless system.
Functional outcome
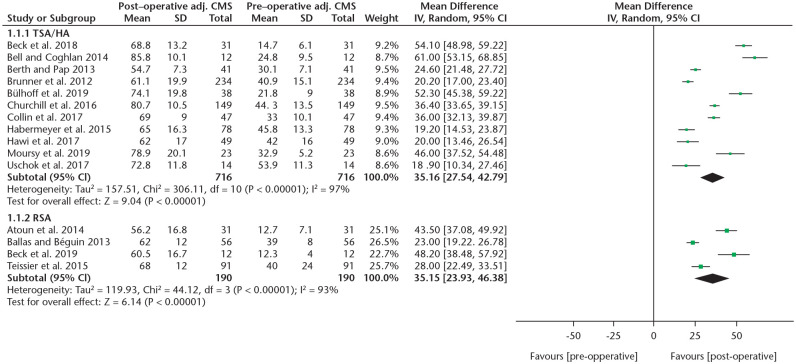
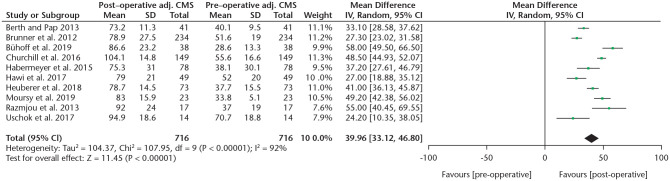
An overview of functional outcome scores and range of motion is provided in Figs. 2 and 3 and Tables 4 and 5.
Fig. 2.
A forest plot showing the absolute CMS increase of TSA/HA and RSA studies.
Note. CMS, Constant-Murley score; TSA, total shoulder arthroplasty; HA, hemi-arthroplasty; RSA, reverse shoulder arthroplasty; IV, inverse variance; CI, confidence interval.
Fig. 3.
A forest plot showing the adjusted CMS increase of TSA/HA studies.
Note. CMS, Constant-Murley score; TSA, total shoulder arthroplasty; HA, hemi-arthroplasty; IV, inverse variance; CI, confidence interval.
Table 4.
Summary of patient-reported outcome measures
| Outcome | No. shoulders (studies) | Postop, WM | Mean gain | P-value | 95% CI of gain |
|---|---|---|---|---|---|
| TSA/HA | |||||
| CMS abs. | 716 (11) | 68.1 | 35.2 | < 0.001 | 27.6, 42.9 |
| CMS adj. | 716 (10) | 84.0 | 40.0 | < 0.001 | 33.1, 46.8 |
| ASES | 369 (5) | 89.2 | 53.5 | < 0.001 | 44.3, 62.7 |
| DASH | 12 (1) | 5.9 | –42.9 | < 0.001 | –51.4, –34.4 |
| QuickDASH | 48 (2) | 20.2 | –41.0 | < 0.001 | –58.7, –23.2 |
| RSA | |||||
| CMS abs. | 190(4) | 63.8 | 35.2 | < 0.001 | 23.9, 46.4 |
| QuickDASH | 28 (2) | 29.3 | –39.9 | < 0.001 | –49.5, –30.4 |
Note. TSA, total shoulder arthroplasty; HA, hemi-arthroplasty; RSA, reverse shoulder arthroplasty; WM, weighted mean.
Table 5.
Combined range of motion results of TSA/HA and RSA studies
| Outcome | No. shoulders (studies) | Postop, WM | Mean gain | P-value | 95% CI of gain |
|---|---|---|---|---|---|
| TSA/HA | |||||
| Elevation | 947 (15) | 137º | 46º | < 0.001 | 39, 53 |
| Abduction | 532 (9) | 122º | 52º | < 0.001 | 42, 62 |
| Ext. rotation | 881 (12) | 44º | 22º | < 0.001 | 17, 27 |
| RSA | |||||
| Elevation | 175 (4) | 139º | 63º | < 0.001 | 49, 77 |
| Abduction | 119 (3) | 132º | 69º | < 0.001 | 47, 91 |
| Ext. rotation | 147 (2) | 41º | 22º | < 0.001 | 4, 41 |
Note. TSA, total shoulder arthroplasty; HA, hemi-arthroplasty; RSA, reverse shoulder arthroplasty; WM, weighted mean; Ext, external.
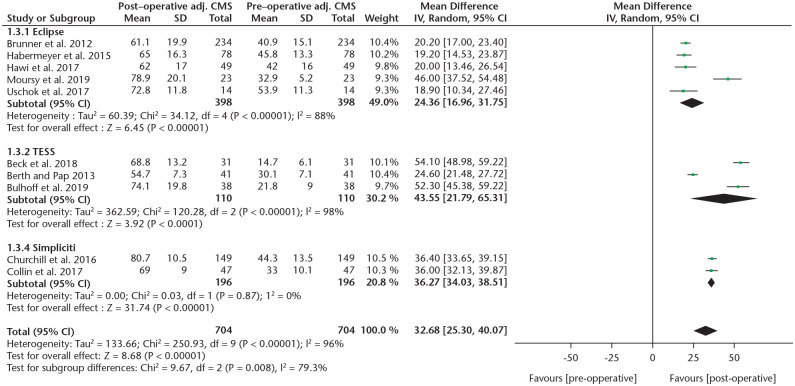
To compare CMS gain between different systems, a subgroup analysis was performed (Fig. 4). Due to limited data, a comparison was only possible between the Eclipse, TESS and Simpliciti systems. A statistically significant higher mean absolute CMS gain was found when comparing the Simpliciti to the Eclipse systems (36 vs. 25, p = 0.006, I2 = 87%). The improvement in the TESS group was 42 points, which was not significantly different compared to the other two types when analysed separately (TESS vs. Simpliciti p = 0.5, TESS vs. Eclipse p = 0.1). The mean adjusted CMS gain was significantly higher comparing the Simpliciti (n = 149) to the Eclipse (n = 431) systems (49% vs. 35%, p = 0.002, I2 = 89.3), although results of the Simpliciti system were provided by only one study.32 No significant differences were found in the ASES score between TSA/HA systems, although data were sparse. DASH scores were reported too infrequently to perform a subgroup analysis, which was also the case for the ASES and DASH scores in RSA studies. In RSA studies the CMS did not show a significant difference between the two systems.
Fig. 4.
A forest plot showing the subgroup analysis of the absolute CMS between different anatomic systems.
Note. CMS, Constant-Murley score; IV, inverse variance; CI, confidence interval.
A comparison between HA results8,35,36 and TSA resu-lts8,9,22,23,32,35,36 did not reveal any significant difference in adjusted CMS (p = 0.42). When comparing studies with short- (mean 2.5 years) to medium-term (mean 7.1 years) follow-up, also no significant difference was found in absolute CMS (p = 0.27) or adjusted CMS (p = 0.52).
A subgroup analysis of range of motion results between all five TSA/HA systems revealed a statistically significant lower increase of forward elevation with the Eclipse compared to the Affinis (31° vs. 62° gain, p = 0.02, I2 = 81.6) and to the Sidus system (31° vs. 52° gain, p = 0.04, I2 = 75.4). External rotation was reported with all systems except the Affinis. In a comparison between these four, the Eclipse performed worse compared to the Sidus system (18° vs. 30° gain, p < 0.001, I2 = 95.7). Other comparisons in forward elevation or external rotation yielded no significant differences. Abduction was only reported for TESS and Eclipse systems and did not show a significant difference. In the RSA group, the range of motion was only sufficiently reported by studies with the TESS RSA system.
Radiographic outcome measures
The most frequently reported radiologic outcomes around the humeral implant were radiolucent lines (RLLs) or osteolysis. The difference between these findings lies in their theoretical aetiology. RLLs are thought to be an early sign of component loosening. Osteolysis can be caused by polyethylene particle disease usually presenting at the bone–implant interface, or by stress shielding usually presenting as decreased bone mineral density at the calcar or greater tubercle.
Radiographic outcomes regarding the humeral component were reported in 19 out of 23 studies in the TSA/HA group (Table 6). RLLs and osteolysis (or stress shielding) around the humeral implant were observed in 7.1% and 7.7% of cases. Humeral component migration was seen in eight out of 1184 cases (0.7%). These eight cases were all reported by Bülhoff et al with TESS implants at a mean follow-up of 3.1 years.31 Of all 382 hemi-arthroplasties, glenoid erosion was seen in 50 cases (13.1%). Superior migration of the humerus, described as a loss of the gothic arch, was observed in 44 of 1184 cases (3.7%).
Table 6.
Incidence rates of humeral radiolucent lines and osteolysis in the TSA/HA group at time of final follow-up, categorized per system
| Implant | No. shoulders (studies) | RLLs | Annual rate | Osteolysis | Annual rate | Follow-up (WM, yrs) |
|---|---|---|---|---|---|---|
| Affinis | 58 (3) | 10 (17.2%) | 4.0% | 9 (15.5%) | 3.6% | 4.3 |
| Eclipse | 571 (7) | 64 (11.2%) | 2.8% | 88 (15.4%) | 3.9% | 3.9 |
| Sidus | 361 (3) | 4 (1.1%) | 0.6% | 4 (1.1%) | 0.6% | 2.0 |
| Simpliciti | 196 (2) | 17 (8.7%) | 3.9% | 0 | – | 2.2 |
| TESS | 148 (4) | 0 | – | 1 (0.7%) | 0.1% | 2.9 |
| Total | 1334 (19) | 95 (7.1%) | 2.1% | 103 (7.7%) | 2% | 2.9 |
Note. TSA, total shoulder arthroplasty; HA, hemi-arthroplasty; RLLs, radiolucent lines; WM, weighted mean; yrs, years.
The presence of progression of osteolysis was reported in nine studies (n = 808). At a mean follow-up of 2.5 years, seven cases of progressive osteolysis were reported (0.9%).6,41 All of these were TSA cases. Of these, four were patients with rheumatoid arthritis and of these, three showed deterioration of clinical outcome, for which one was revised.41 No implant loosening was seen intraoperatively. Apart from these three cases, none of the other studies reported a correlation of their radiologic findings with functional outcome.
In RSA literature, only three articles observed radiologic abnormalities around the humeral implant. Osteolysis was reported in one case,44 radiolucent lines in three cases,47 and migration of the humeral component was seen in one case which did not require revision.45 Glenoid notching was observed in all articles, with an overall incidence of 18.4% (67 out of 353 cases) at a mean follow-up of 3.8 years. Grade 1 or 2 was seen in 51 cases (76.1%), Grade 3 in three (4.5%). No Grade 4 notching was found, and in 13 cases grades were not specified (19.4%). No correlation to outcome was found. No significant difference in notching between the Verso and TESS systems was found (18.1% vs. 18.6%, p = 0.89).
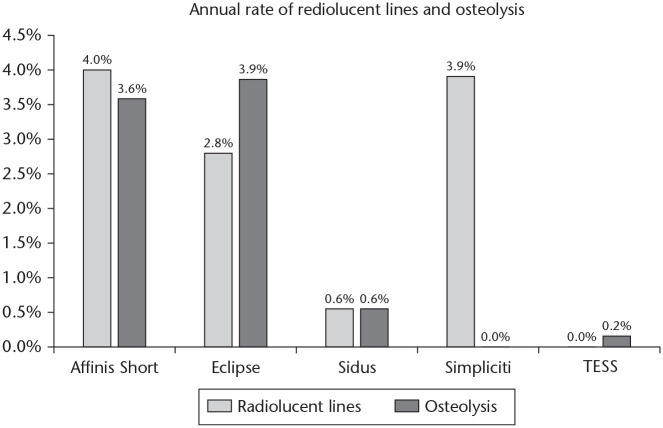
When comparing the incidence of RLLs between systems corrected for follow-up, the annual rates of RLLs reported with the Affinis, Eclipse and Simpliciti systems were significantly higher than the rates reported with the TESS or Sidus systems (p < 0.05) (Fig. 5). Differences between the Sidus and TESS, and between the Affinis, Eclipse and Simpliciti systems were not significant (p > 0.05).
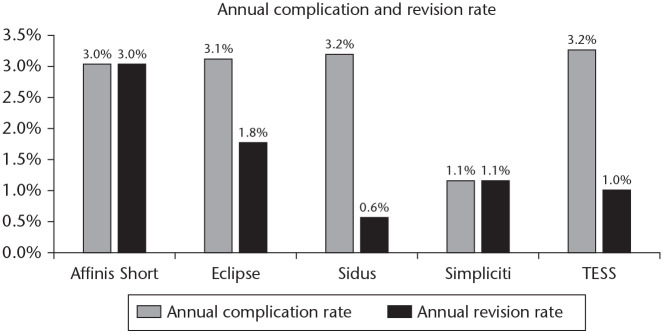
Fig. 5.
Annual rates of humeral component radiolucent lines and osteolysis, categorized by type of implant.
When comparing the incidence of reported humeral osteolysis between systems corrected for follow-up, the annual rates of osteolysis reported with the Affinis and Eclipse systems were significantly higher compared to the rates reported with the Sidus, TESS or Simpliciti systems (p < 0.05). There was no significant difference between the Affinis and the Eclipse systems (p = 0.99). Also, no significant difference was found in incidence between the Sidus, TESS or Simpliciti systems (p > 0.05).
Complications/revisions
The overall complication rate in the TSA/HA group was 9.7% at a mean follow-up of 3.0 years (Table 7). A total of nine humeral-component-related complications were reported (0.6%). Two cases of humeral loosening were described (Sidus, Eclipse), of which one was revised (Sidus).30,42 Seven were intraoperative fractures (5 TESS, 2 Sidus), which were treated conservatively.37,38,42 A total of 93 reoperations were performed (6.0%). Of these, 79 were revisions (5.1%).
Table 7.
An overview of all complications and reoperations in the stemless TSA/HA group
| Complication | No. cases | Percentage overall | Percentage of all complications | Reoperations | Percentage overall | Percentage of all revisions |
|---|---|---|---|---|---|---|
| Humeral component related | ||||||
| Humeral loosening | 2 | 0.1% | 1% | 1 | 0.1% | 1% |
| Humeral i.o. fracture | 7 | 0.5% | 5% | – | ||
| Glenoid component related§ | ||||||
| Glenoid loosening | 12 | 1.1%§ | 8% | 8 | 0.7%§ | 10% |
| Glenoid i.o. fracture | 8 | 0.7%§ | 5% | – | ||
| Disassembly glenoid liner | 1 | 0.1%§ | 1% | 1 | 0.1%§ | 1% |
| Hemi-arthroplasty related‡ | ||||||
| Sympt. glenoid erosion | 12 | 3.1%‡ | 8% | 12 | 3.1%‡ | 15% |
| General complications | ||||||
| Cuff insufficiency | 53 | 3.7% | 35% | 33 rev/3 tt | 2.3%/0.2% | 42% |
| Infection | 19 | 1.2% | 13% | 18 | 1.2% | 23% |
| Postop traumatic fracture | 7 | 0.5% | 5% | 3 rev/2 ORIF | 0.2%/0.1% | 4% |
| Frozen shoulder | 5 | 0.3% | 3% | 3 | 0.2% | |
| Instability | 2 | 0.1% | 1% | 2 | 0.1% | 3% |
| Biceps tendonitis | 3 | 0.2% | 2% | 3 | 0.2% | |
| Undefined pain | 3 | 0.2% | 2% | 1 | 0.1% | 1% |
| Painful os acromiale | 1 | 0.1% | 1% | 1 | 0.1% | |
| Hematoma | 1 | 0.1% | 1% | 1 | 0.1% | |
| Thrombosis | 1 | 0.1% | 1% | – | ||
| Temp. neuropraxia | 7 | 0.5% | 5% | – | ||
| Other | 6 | 0.4% | 4% | – | ||
| Total | 150 | 9.7% | 92 | 6.0% | ||
Note. TSA, total shoulder arthroplasty; HA, hemi-arthroplasty; No., number; i.o., intraoperative; sympt., symptomatic; temp., temporary; rev, revision arthroplasty; tt, tendon transfer; ORIF, open reduction internal fixation.
Rates are presented as a percentage of total number TSA implants. ‡Rate is presented as a percentage of total HA implants.
In the RSA group, the overall complication rate was 13.7% (50 of 365 cases) at a mean follow-up of 3.8 years (Table 8). Humeral-component-related complications were observed in six cases (1.6%). Two were loosening of TESS implants reported by Kadum et al in the first postoperative week, which may be due to malpositioning.24 Four cases were intraoperative non-displaced fractures during implantation of a Verso implant, which were treated conservatively.43,46 Of the 26 reoperations, a total of 21 were revision cases (5.8%).
Table 8.
An overview of all complications and reoperations in the stemless RSA group.
| Complication | No. cases | Percentage overall | Percentage of all complications | Reoperations | Percentage overall | Percentage of all revisions |
|---|---|---|---|---|---|---|
| Humeral component related | ||||||
| Humeral loosening | 2 | 0.5% | 4% | 2 | 0.5% | 10% |
| Humeral i.o. fracture | 4 | 1.1% | 8% | 0 | – | |
| Glenoid component related | ||||||
| Glenoid loosening | 2 | 0.5% | 4% | 2 | 0.5% | 10% |
| Glenoid i.o. fracture | 2 | 0.5% | 4% | 0 | – | |
| Disassembly glenosphere | 7 | 1.9% | 14% | 7 | 1.9% | 33% |
| General complications | ||||||
| Post-op traumatic fracture | 14 | 3.8% | 28% | 3 | 0.8% | 14% |
| Scapular stress fracture | 5 | 1.4% | 10% | 1 | 0.3% | |
| Instability | 8 | 2.2% | 16% | 8 (6 rev) | 2.2% (1.6%) | 29% |
| Infection | 1 | 0.3% | 2% | 1 | 0.3% | 5% |
| Frozen shoulder | 3 | 0.8% | 6% | 0 | – | |
| Hematoma | 1 | 0.3% | 2% | 1 | 0.3% | |
| Painful os acromiale | 1 | 0.3% | 2% | 1 | 0.3% | |
| Total | 50 | 13.6% | 26 | 7.1% | ||
Note. RSA, reverse shoulder arthroplasty; No., number; i.o., intraoperative; temp, temporary.
To compare complication and revision rates between systems, a subgroup analysis corrected for follow-up was performed. In the TSA/HA group, the annual complication rate was significantly lower with the Simpliciti system compared to the Eclipse, Sidus or TESS systems (p = 0.01; p = 0.04; p = 0.03) (Fig. 6). No other comparison revealed a significant difference. When comparing revision rates, the Sidus system had a significantly lower annual rate compared to the Affinis or Eclipse systems (p = 0.03; p = 0.02) (Fig. 6). In the RSA group no significant differences were found in annual complication and revision rates between the two systems (p > 0.05).
Fig. 6.
Annual complication and revision rates, categorized by type of implant.
Discussion
This systematic review of 31 included studies reporting data on a total of 1,944 shoulders provides an overview of all types of stemless shoulder arthroplasty. The findings of this review show good clinical results in the short and medium term in stemless hemi-, total or reverse shoulder arthroplasty. All reported systems performed well, although according to our subgroup analysis some differences were seen which may be attributed to differences in baseline characteristics and high study heterogeneity.
Clinical outcome
Functional results in the short- and medium-term follow-up were overall good for the TSA/HA as well as the RSA group as improvement of the CMS exceeded both the minimal clinically important difference (MCID)50,51 and the substantial clinical benefit (SCB).54 This was also the case for the ASES in the TSA/HA group, as well as the range of motion in all three directions in both groups.50,52
The seven included comparative studies, of which two were RCTs, found no significant clinical differences when compared to conventional stemmed anatomic and reverse shoulder arthroplasty.9,22–24,26,47,53 These results confirm the overall results of this review, which resemble those of conventional third-generation stemmed anatomic54–58 and reverse shoulder arthroplasty.59,60
Radiographic outcomes
Radiographic abnormalities are of interest, as they possibly influence outcome4 and may be a predictor for aseptic loosening as is seen in hip61 or knee62 arthroplasty. Bell et al25 compared osteolysis rates of 23 stemless TSAs with 39 stemmed TSAs at 5.5 years follow-up and found a significantly higher rate of osteolysis in the stemmed group (p = 0.005). However, clinical outcome was not worse in the stemmed group.
Osteolysis rates at the proximal humerus in conventional stemmed TSAs are reported between 23% and 63%.4,54,57,63,64 These rates are higher compared to the results reported in this review, although follow-up is longer in some of these stemmed studies. Nevertheless, the lower rates in this study are in line with the finite element analysis by Razfar et al.65 They found significantly less cortical bone stress in their model when using traditional stemmed TSAs compared to stemless and short-stemmed TSAs. Additionally, they found significantly higher trabecular bone stresses in stemless compared to stemmed TSAs.65 Both suggesting a lesser probability of stress shielding in stemless designs. Furthermore, higher metaphyseal volumetric fill ratios are found to have a significant correlation to stress shielding, suggesting that less filling of the metaphysis results in less stress shielding.57,66 This could be an explanation behind the lower rates of radiologic abnormalities seen in this stemless series.
The incidence of radiolucent lines or osteolysis in the RSA group was very low, both reported in < 1% of cases. Glenoid notching was found in an overall 18.4% of cases at a mean 3.8 years of follow-up. Rates of notching in conventional stemmed RSAs vary widely, with rates reported from 4.6% up to 96% in the literature.67 Notching is a multifactorial problem, which appears to have a correlation to function.68,69 It is unclear what the role of the stemless component has in this low rate, as glenoid component positioning is most likely a more important factor.67 A possible theory could be the flexibility in positioning of the humeral component, which could result in a lower neck shaft angle, resulting in lower notching rates. Nevertheless, these relatively low rates are promising.
Complications
Reported overall complication rates were low, with a total rate of 9.8% of TSA/HA and 13.7% of RSA cases. Only a few were related to the humeral component, with a reported 0.6% and 1.6% of cases respectively. In a comprehensive review, Bohsali et al reported an overall complication rate of 10.3% of stemmed TSAs and 16.1% of stemmed RSAs, and a loosening rate of 0.1% of TSAs and 0.7% of RSAs.70 These are all comparable to the rates in this series.
The loosening rate in the TSA/HA group is, however, much lower compared to the series of Gonzalez et al (6% of TSAs and HAs), which can be explained by the high loosening rates of uncemented stems in several studies included in their series (up to 48.7%).1
RSA overall complication rates were lower in this series compared to the 24% overall rate Zumstein et al found in their review of 782 stemmed RSAs.71 This can be explained by the difference in indication, as stemless RSA was placed predominantly in CTA patients (71%), and only 6% were revision cases.
Interestingly, the instability rate of 2.0% of stemless RSAs in this series is significantly lower than the rates reported in the literature (4.7%71 to 5%70). Since many different patient-, implant- or technique-related factors influence stability, it is unclear what precisely causes the lower rate in this review. As stated before, a possible reason could be the difference in indications in this study compared to other reviews.
Another notable difference compared to stemmed implants was the incidence of intraoperative humeral fractures. The rate in the TSA/HA group was 0.5%, compared to 1.9%1 or 2.3%70 in the literature. In the RSA group the rate was 1.1%, versus 3.0% found by Zumstein et al.71 It can be assumed that the absence of medullary preparation and the implantation of a substantially smaller humeral implant is the cause of this lower rate.
Cuff insufficiency or secondary tears were higher in the present study compared to stemmed TSA literature (3.7% versus 0.9%72 or 2.7%1). This is primarily caused by the relatively high rate in four studies (overall 9.6%),8,34–36 which can be partly explained by the indications, as cuff tear arthropathy or cuff tears were not considered a contraindication in two of these.35,36
Infection rates in this series are comparable to those reported in the literature (1.2% versus 0.5–1.1%).1,70,72 However, the majority of infections (10) were reported by Johansson et al in their retrospective cohort of 102 Eclipse implants.49 They found a significantly higher rate compared to conventional stemmed arthroplasty. Although baseline characteristics of the two cohorts were not similar (more male and lower age in the stemless group), it was unclear what caused their unusually high rate of infections (9.8%).
Revisions
The overall revision rate was 5.1% in the TSA/HA group at three years follow-up. The most common reasons in the TSA/HA group were rotator cuff failure (2.5%), infection (1.2%) and symptomatic glenoid erosion (3.1%). In a registry study by Dillon et al with a mean follow-up of 3.3 years including 3,026 TSAs and 2,179 HAs, the overall revision rate of stemmed TSA was 2.1% and of HA 6.2%.72 When combined, their rate was 3.2%, which is lower compared to this series. However, compared to the TSA/HA series of Gonzalez et al, overall revision rates in this series are lower (5.1% vs. 7.9%).1 They found glenoid component loosening the most frequent reason for revision in TSAs, which is most likely due to their higher mean follow-up of six years. Revision rates for glenoid erosion vary in the literature, with rates reported from 1.7%72 to 6.2%.1 The rate of 3.1% in this series is therefore comparable to the literature.
In the RSA group the overall revision rate was 5.8% at 3.6 years follow-up. The most common reasons were glenosphere disassembly (1.9%), instability (1.6%) and periprosthetic postoperative fracture (0.8%). This overall rate is lower compared to the series of Zumstein et al (10.1%), which can be explained by the much higher percentage of revision cases in their series (28%).71 The rate is similar to the rate reported in the study of Dillon et al (5.1%). Glenosphere disassembly occurred relatively frequent, with a total of seven cases. However, in three cases it was caused by trauma45(Verso), another three cases were caused by difficulty implanting the glenoid component44 (TESS), which did not occur after a glenoid design modification.
Implant comparisons
Compared to the Simpliciti and TESS, the Eclipse seems to have significantly lower outcomes in CMS and range of motion. As baseline characteristics between studies are substantially different, it is not possible to draw strong conclusions from this analysis. For instance, the Eclipse group has a much higher rate of included HAs (43% vs. 4% in the Simpliciti and 28% in the TESS group). Although our subgroup analysis comparing HA and TSA did not reveal significant differences, it has been reported in the literature that HA performs worse in clinical outcome compared to TSA.73,74 Krukenberg et al also found a significantly greater increase of CMS, ASES and range of motion in 73 TSAs versus 32 HAs using the Sidus system in osteoarthritis patients.38 However, no information was given regarding baseline characteristics, and the study was performed at nine different centres with potential differences in evaluation and in indications for HA and TSA. Due to incomplete data, this study was not included in the subgroup analysis.
When comparing radiologic results between systems, annual incidence of RLLs was significantly higher with the Affinis, Eclipse and Simpliciti compared to the Sidus and TESS systems. Annual osteolysis rates were significantly higher in the Affinis and Eclipse systems compared to the other three systems.
The results of this subgroup analysis were partly confirmed by the finite element analysis carried out by Comenda et al.75 In their study they compared bone resorption rates between different stemless implant designs. The Eclipse system performed worse with regards to bone resorption and adaptation compared to the Simpliciti and Sidus systems.75 The other systems were not included in their analysis.
In the comparison of complication rates between systems, the Simpliciti performed better compared to the Eclipse, Sidus and TESS systems. The majority of stemless shoulders in the Simpliciti series were described by the well-designed prospective case series of Churchill et al.32 They used relatively strict inclusion criteria, which may be the reason for their low complication rate. The Sidus had a lower annual revision rate compared to the Affinis and Eclipse systems. A possible reason could be the lower rate of HAs included in the Sidus cohort (9% vs. 27% and 43%), which can be the cause of lower revision rates.73,76 However, when not including revision cases for glenoid erosion, the difference between these systems was still significant. Another reason could be the overall high rate of primary osteoarthritis patients in the Sidus group compared to the other groups, 98% vs. 54% and 57% respectively, as substantial differences in indications could influence revision rates.
Strengths and limitations
The strength of this study lies in its methodology. Most of the steps were performed by two reviewers, independently. Furthermore, only studies with results of two years follow-up or longer are included, which avoids a possible negative influence on results by inclusion of very short-term data. Another strength is the subgroup analysis, which provides new information regarding differences between systems.
The first limitation of this study lies in the fusion of TSA and HA results, since most of the included articles did not distinguish between TSA and HA results. As moderate evidence shows that results of HA might be inferior to TSA,73 combining these results could negatively influence TSA results. It should also be noted that substantial differences in baseline characteristics between TSA and HA patients commonly exist, such as age, osteoarthritis grade, indication, etc. In an attempt to analyse a difference in outcome between these two types, we performed a subgroup analysis comparing stemless TSA versus HA results. No significant difference in CMS or ROM was found, although baseline characteristics between these groups were not provided or were very sparse. Therefore, these results should be interpreted with caution. The second limitation of this review lies in the differences of design in stemless systems. This makes an overview of results less reliable, as every feature might have its own possible advantage or disadvantage. In the current literature, it is not possible to assess the influence of these features independently. The third limitation is high study heterogeneity, with substantial differences in baseline characteristics, which makes comparisons between studies or systems less reliable. Additionally, most are single-surgeon series, with differences in indication and approach. Most of these studies have a low quality of evidence, with a short overall mean follow-up of 3.2 years, and with the majority having a high risk of bias. Additionally, five out of eight RSA studies and 13 out of 23 studies report a possible conflict of interest, although a subgroup analysis did not show superior results comparing absolute CMS and forward elevation in studies with a possible conflict of interest to studies without. Another weakness are the sparse data regarding different patient- and surgeon-reported outcomes, and the wide array of different scores used in studies. From a total of nine different outcome scores, the CMS was described most, followed by the ASES score. The CMS is the only outcome score that includes an objective strength and function measurement. Although mentioned in one table with other patient-reported outcome scores, the results of the CMS should not be interpreted as such. With regard to the subgroup analyses, the most important weakness is the difference in follow-up between systems. Outcomes deteriorate over time. In an attempt to assess differences between short- and medium-term follow-up, an analysis was performed which revealed no significant differences in CMS. Although this comparison was performed across different systems, and high heterogeneity was present. We used annual rates to compare radiologic outcomes and complication and revision rates between systems. It is important to note that most findings do not have a linear correlation with time. For instance, rates of radiologic abnormalities around the humeral implant are higher with longer follow-up, but their first appearance can take up to four years.4
Conclusion
This systematic review and meta-analysis of 31 studies with a total of 1,944 stemless TSA, HA or RSA implants, shows good functional results in the short- and medium-term follow-up. When comparing these results to conventional stemmed shoulder arthroplasty literature, similar functional outcome, complication and revision rates were found. Radiologic outcomes are better when compared to stemmed implants, although a correlation to improved clinical outcomes has to be shown. Subgroup analyses revealed significant differences between stemless systems, although firm conclusions cannot be drawn, as the quality of the evidence was low with high study heterogeneity and substantial differences in baseline characteristics and follow-up. Therefore, this review found no clinical advantage of stemless over conventional systems in the short- and medium-term follow-up, although there might be an advantage during revision surgery. Although these results are promising, there is still a need for well-designed long-term randomized trials with sufficient power in order to assess the superiority of stemless over conventional arthroplasty, and of one design over another. Additionally, a well-designed study is needed to analyse the success of revision after primary stemmed versus stemless designs.
Footnotes
ICMJE Conflict of interest statement: AVN reports consultancy for DePuy Synthes and Link Lima, and educational efforts and side to side teaching in the operating room.
The other authors declare no conflict of interest relevant to this work.
OA licence text: This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Gonzalez JF, Alami GB, Baque F, Walch G, Boileau P. Complications of unconstrained shoulder prostheses. J Shoulder Elbow Surg 2011;20:666–682. [DOI] [PubMed] [Google Scholar]
- 2. Denard PJ, Raiss P, Gobezie R, Edwards TB, Lederman E. Stress shielding of the humerus in press-fit anatomic shoulder arthroplasty: review and recommendations for evaluation. J Shoulder Elbow Surg 2018;27:1139–1147. [DOI] [PubMed] [Google Scholar]
- 3. Raiss P, Schnetzke M, Wittmann T, et al. Postoperative radiographic findings of an uncemented convertible short stem for anatomic and reverse shoulder arthroplasty. J Shoulder Elbow Surg 2019;28:715–723. [DOI] [PubMed] [Google Scholar]
- 4. Raiss P, Edwards TB, Deutsch A, et al. Radiographic changes around humeral components in shoulder arthroplasty. J Bone Jt Surg – Ser A 2014;96:e54. [DOI] [PubMed] [Google Scholar]
- 5. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop 2010;34:517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ballas R, Teissier P, Teissier J. Stemless shoulder prosthesis for treatment of proximal humeral malunion does not require tuberosity osteotomy. Int Orthop 2016;40:1473–1479. [DOI] [PubMed] [Google Scholar]
- 7. Holschen M, Franetzki B, Witt KA, Liem D, Steinbeck J. Is reverse total shoulder arthroplasty a feasible treatment option for failed shoulder arthroplasty? A retrospective study of 44 cases with special regards to stemless and stemmed primary implants. Musculoskelet Surg 2017;101:173–180. [DOI] [PubMed] [Google Scholar]
- 8. Heuberer PR, Brandl G, Pauzenberger L, Laky B, Kriegleder B, Anderl W. Radiological changes do not influence clinical mid-term outcome in stemless humeral head replacements with hollow screw fixation: a prospective radiological and clinical evaluation. BMC Musculoskelet Disord 2018;19:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berth A, Pap G. Stemless shoulder prosthesis versus conventional anatomic shoulder prosthesis in patients with osteoarthritis: a comparison of the functional outcome after a minimum of two years follow-up. J Orthop Traumatol 2013;14:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. iDataResearch, Small bone and joint devices market analysis, size, trends | Europe | 2018–2024. Burnaby, BN, Canada: 2018. [Google Scholar]
- 11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003;73:712–716. [DOI] [PubMed] [Google Scholar]
- 13. Constant CR, Murley AHG. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res 1987;214:160–164. [PubMed] [Google Scholar]
- 14. Katolik LI, Romeo AA, Cole BJ, Verma NN, Hayden JK, Bach BR. Normalization of the Constant score. J Shoulder Elbow Surg 2005;14:279–285. [DOI] [PubMed] [Google Scholar]
- 15. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg 1994;3:347–352. [DOI] [PubMed] [Google Scholar]
- 16. Solway S, Beaton DE, McConnell SB. C: the DASH outcome measure user’s manual. Second ed Toronto: Ontario Inst Work Heal, 2002:5. [Google Scholar]
- 17. Beaton DE, Wright JG, Katz JN, et al. Development of the QuickDASH: comparison of three item-reduction approaches. J Bone Jt Surg – Ser A 2005;87:1038–1046. [DOI] [PubMed] [Google Scholar]
- 18. Audigé L, Schwyzer HK, Durchholz H; Shoulder Arthroplasty Core Event Set (SA CES) Consensus Panel. Core set of unfavorable events of shoulder arthroplasty: an international Delphi consensus process. J Shoulder Elbow Surg 2019;28:2061–2071. [DOI] [PubMed] [Google Scholar]
- 19. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walter SD, Yao X. Effect sizes can be calculated for studies reporting ranges for outcome variables in systematic reviews. J Clin Epidemiol 2007;60:849–852. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uschok S, Magosch P, Moe M, Lichtenberg S, Habermeyer P. Is the stemless humeral head replacement clinically and radiographically a secure equivalent to standard stem humeral head replacement in the long-term follow-up? A prospective randomized trial. J Shoulder Elbow Surg 2017;26:225–232. [DOI] [PubMed] [Google Scholar]
- 23. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg 2013;22:206–214. [DOI] [PubMed] [Google Scholar]
- 24. Kadum B, Mukka S, Englund E, Sayed-Noor A, Sjödén G. Clinical and radiological outcome of the Total Evolutive Shoulder System (TESS®) reverse shoulder arthroplasty: a prospective comparative non-randomised study. Int Orthop 2014;38:1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bell SN, Christmas MUSI, Coghlan JA. Proximal humeral osteolysis and glenoid radiolucent lines in an anatomic shoulder arthroplasty: a comparison of a ceramic and a metal humeral head component. J Shoulder Elb Surg 2019. doi: 10.1016/j.jse.2019.09.032 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 26. Spranz DM, Bruttel H, Wolf SI, Zeifang F, Maier MW. Functional midterm follow-up comparison of stemless total shoulder prostheses versus conventional stemmed anatomic shoulder prostheses using a 3D-motion-analysis. BMC Musculoskelet Disord 2017;18:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aibinder WR, Bicknell RT, Bartsch S, Scheibel M, Athwal GS. Subscapularis management in stemless total shoulder arthroplasty: tenotomy versus peel versus lesser tuberosity osteotomy. J Shoulder Elbow Surg 2019;28:1942–1947. [DOI] [PubMed] [Google Scholar]
- 28. Beck S, Beck V, Wegner A, Dudda M, Patsalis T, Jäger M. Long-term survivorship of stemless anatomical shoulder replacement. Int Orthop 2018;42:1327–1330. [DOI] [PubMed] [Google Scholar]
- 29. Beck S, Patsalis T, Busch A, et al. Long-term results of the reverse Total Evolutive Shoulder System (TESS). Arch Orthop Trauma Surg 2019;139:1039–1044. [DOI] [PubMed] [Google Scholar]
- 30. Brunner UH, Fruth M, Rückl K, et al. Die schaftfreie Eclipse-Prothese - Indikation und mittelfristige Ergebnisse: eine prospektive Multicenterstudie. Obere Extrem 2012;7:22–28. [Google Scholar]
- 31. Bülhoff M, Spranz D, Maier M, Raiss P, Bruckner T, Zeifang F. Mid-term results with an anatomic stemless shoulder prosthesis in patients with primary osteoarthritis. Acta Orthop Traumatol Turc 2019;53:170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Churchill RS, Chuinard C, Wiater JM, et al. Clinical and radiographic outcomes of the Simpliciti canal-sparing shoulder arthroplasty system. J Bone Joint Surg Am 2016;98:552–560. [DOI] [PubMed] [Google Scholar]
- 33. Collin P, Matsukawa T, Boileau P, Brunner U, Walch G. Is the humeral stem useful in anatomic total shoulder arthroplasty? Int Orthop 2017;41:1035–1039. [DOI] [PubMed] [Google Scholar]
- 34. Gallacher S, Williams HLM, King A, Kitson J, Smith CD, Thomas WJ. Clinical and radiologic outcomes following total shoulder arthroplasty using Arthrex Eclipse stemless humeral component with minimum 2 years’ follow-up. J Shoulder Elbow Surg 2018;27:2191–2197. [DOI] [PubMed] [Google Scholar]
- 35. Habermeyer P, Lichtenberg S, Tauber M, Magosch P. Midterm results of stemless shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg 2015;24:1463–1472. [DOI] [PubMed] [Google Scholar]
- 36. Hawi N, Magosch P, Tauber M, Lichtenberg S, Habermeyer P. Nine-year outcome after anatomic stemless shoulder prosthesis: clinical and radiologic results. J Shoulder Elbow Surg 2017;26:1609–1615. [DOI] [PubMed] [Google Scholar]
- 37. Huguet D, DeClercq G, Rio B, Teissier J, Zipoli B; TESS Group. Results of a new stemless shoulder prosthesis: radiologic proof of maintained fixation and stability after a minimum of three years’ follow-up. J Shoulder Elbow Surg 2010;19:847–852. [DOI] [PubMed] [Google Scholar]
- 38. Krukenberg A, Imiolczyk JP, Moroder P, Scheibel M. [Shoulder arthroplasty]. Z Orthop Unfall 2018;156:227–238. [DOI] [PubMed] [Google Scholar]
- 39. von Engelhardt LV, Manzke M, Breil-Wirth A, Filler TJ, Jerosch J. Restoration of the joint geometry and outcome after stemless TESS shoulder arthroplasty. World J Orthop 2017;8:790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moursy M, Niks M, Kadavkolan AS, Lehmann LJ. Do the radiological changes seen at mid term follow up of stemless shoulder prosthesis affect outcome? BMC Musculoskelet Disord 2019;20:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jordan RW, Manoharan G, Van Liefland M, et al. Reliability of stemless shoulder arthroplasty in rheumatoid arthritis: observation of early lysis around the humeral component. Musculoskelet Surg. 2019. doi: 10.1007/s12306-019-00629-8 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 42. Athwal GS, Krupp RJ, Carlson G, Bicknell RT. A multicenter, prospective 2-year analysis of the Sidus stem-free shoulder arthroplasty system. JSES Int 2019;4:120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Atoun E, Van Tongel A, Hous N, et al. Reverse shoulder arthroplasty with a short metaphyseal humeral stem. Int Orthop 2014;38:1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ballas R, Béguin L. Results of a stemless reverse shoulder prosthesis at more than 58 months mean without loosening. J Shoulder Elbow Surg 2013;22:e1–e6. [DOI] [PubMed] [Google Scholar]
- 45. Leonidou A, Virani S, Buckle C, Yeoh C, Relwani J. Reverse shoulder arthroplasty with a cementless short metaphyseal humeral prosthesis without a stem: survivorship, early to mid-term clinical and radiological outcomes in a prospective study from an independent centre. Eur J Orthop Surg Traumatol 2020;30:89–96. [DOI] [PubMed] [Google Scholar]
- 46. Levy O, Narvani A, Hous N, et al. Reverse shoulder arthroplasty with a cementless short metaphyseal humeral implant without a stem: clinical and radiologic outcomes in prospective 2- to 7-year follow-up study. J Shoulder Elbow Surg 2016;25:1362–1370. [DOI] [PubMed] [Google Scholar]
- 47. Moroder P, Ernstbrunner L, Zweiger C, et al. Short to mid-term results of stemless reverse shoulder arthroplasty in a selected patient population compared to a matched control group with stem. Int Orthop 2016;40:2115–2120. [DOI] [PubMed] [Google Scholar]
- 48. Teissier P, Teissier J, Kouyoumdjian P, Asencio G. The TESS reverse shoulder arthroplasty without a stem in the treatment of cuff-deficient shoulder conditions: clinical and radiographic results. J Shoulder Elbow Surg 2015;24:45–51. [DOI] [PubMed] [Google Scholar]
- 49. Johansson L, Hailer NP, Rahme H. High incidence of periprosthetic joint infection with propionibacterium acnes after the use of a stemless shoulder prosthesis with metaphyseal screw fixation – a retrospective cohort study of 241 patients. BMC Musculoskelet Disord 2017;18:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Simovitch R, Flurin PH, Wright T, Zuckerman JD, Roche CP. Quantifying success after total shoulder arthroplasty: the minimal clinically important difference. J Shoulder Elbow Surg 2018;27:298–305. [DOI] [PubMed] [Google Scholar]
- 51. Torrens C, Guirro P, Santana F. The minimal clinically important difference for function and strength in patients undergoing reverse shoulder arthroplasty. J Shoulder Elbow Surg 2016;25:262–268. [DOI] [PubMed] [Google Scholar]
- 52. Simovitch R, Flurin PH, Wright T, Zuckerman JD, Roche CP. Quantifying success after total shoulder arthroplasty: the substantial clinical benefit. J Shoulder Elbow Surg 2018;27:903–911. [DOI] [PubMed] [Google Scholar]
- 53. Bell SN, Coghlan JA. Short stem shoulder replacement. Int J Shoulder Surg 2014;8:72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Levy JC, Berglund D, Vakharia R, et al. Midterm results of anatomic total shoulder arthroplasty with a third-generation implant. J Shoulder Elbow Surg 2019;28:698–705. [DOI] [PubMed] [Google Scholar]
- 55. Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Jt Surg – Ser B 2009;91:1594–1600. [DOI] [PubMed] [Google Scholar]
- 56. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Jt Surg – Ser A 2012;94:1–10. [DOI] [PubMed] [Google Scholar]
- 57. Schnetzke M, Rick S, Raiss P, Walch G, Loew M. Mid-term results of anatomical total shoulder arthroplasty for primary osteoarthritis using a short-stemmed cementless humeral component. Bone Joint J 2018;100-B:603–609. [DOI] [PubMed] [Google Scholar]
- 58. Pfahler M, Meier K, Anetzberger H, Schulz CU. An evaluation of the efficiency of the use of an anatomical third generation shoulder prosthesis in 102 patients. Acta Chir Belg 2009;109:86–92. [DOI] [PubMed] [Google Scholar]
- 59. Sevivas N, Ferreira N, Andrade R, et al. Reverse shoulder arthroplasty for irreparable massive rotator cuff tears: a systematic review with meta-analysis and meta-regression. J Shoulder Elbow Surg 2017;26:e265–e277. [DOI] [PubMed] [Google Scholar]
- 60. Petrillo S, Longo UG, Papalia R, Denaro V. Reverse shoulder arthroplasty for massive irreparable rotator cuff tears and cuff tear arthropathy: a systematic review. Musculoskelet Surg 2017;101:105–112. [DOI] [PubMed] [Google Scholar]
- 61. Pajarinen J, Gallo J, Takagi M, Goodman SB, Mjöberg B. Particle disease really does exist. Acta Orthop 2018;89:133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Evans JT, Walker RW, Evans JP, Blom AW, Sayers A, Whitehouse MR. How long does a knee replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet 2019;393:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Denard PJ, Noyes MP, Walker JB, et al. Proximal stress shielding is decreased with a short stem compared with a traditional-length stem in total shoulder arthroplasty. J Shoulder Elbow Surg 2018;27:53–58. [DOI] [PubMed] [Google Scholar]
- 64. Verborgt O, El-Abiad R, Gazielly DF. Long-term results of uncemented humeral components in shoulder arthroplasty. J Shoulder Elbow Surg 2007;16:S13–S18. [DOI] [PubMed] [Google Scholar]
- 65. Razfar N, Reeves JM, Langohr DG, Willing R, Athwal GS, Johnson JA. Comparison of proximal humeral bone stresses between stemless, short stem, and standard stem length: a finite element analysis. J Shoulder Elbow Surg 2016;25:1076–1083. [DOI] [PubMed] [Google Scholar]
- 66. Celik H, Chauhan A, Flores-Hernandez C, DʼLima D, Hoenecke H. Three-dimensional volumetric filling ratio predicts stress shielding in short-stem anatomic total shoulder arthroplasty. J Am Acad Orthop Surg 2020;29:e163–e166. [DOI] [PubMed] [Google Scholar]
- 67. Friedman RJ, Barcel DA, Eichinger JK. Scapular notching in reverse total shoulder arthroplasty. J Am Acad Orthop Surg 2019;27:200–209. [DOI] [PubMed] [Google Scholar]
- 68. Mollon B, Mahure SA, Roche CP, Zuckerman JD. Impact of scapular notching on clinical outcomes after reverse total shoulder arthroplasty: an analysis of 476 shoulders. J Shoulder Elbow Surg 2017;26:1253–1261. [DOI] [PubMed] [Google Scholar]
- 69. Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the delta III reverse total shoulder replacement. J Bone Jt Surg – Ser A 2007;89:588–600. [DOI] [PubMed] [Google Scholar]
- 70. Bohsali KI, Bois AJ, Wirth MA. Complications of shoulder arthroplasty. J Bone Joint Surg Am 2017;99:256–269. [DOI] [PubMed] [Google Scholar]
- 71. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2011;20:146–157. [DOI] [PubMed] [Google Scholar]
- 72. Dillon MT, Ake CF, Burke MF, et al. The Kaiser Permanente shoulder arthroplasty registry: results from 6,336 primary shoulder arthroplasties. Acta Orthop 2015;86:286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Singh JA, Sperling J, Buchbinder R. Surgery for shoulder osteoarthritis. Cochrane Database Syst Rev 2010;10. [DOI] [PubMed] [Google Scholar]
- 74. Garcia GH, Liu JN, Mahony GT, et al. Hemiarthroplasty versus total shoulder arthroplasty for shoulder osteoarthritis: a matched comparison of return to sports. Am J Sports Med 2016;44:1417–1422. [DOI] [PubMed] [Google Scholar]
- 75. Comenda M, Quental C, Folgado J, Sarmento M, Monteiro J. Bone adaptation impact of stemless shoulder implants: a computational analysis. J Shoulder Elbow Surg 2019;28:1886–1896. [DOI] [PubMed] [Google Scholar]
- 76. van den Bekerom MP, Geervliet PC, Somford MP, van den Borne MP, Boer R. Total shoulder arthroplasty versus hemiarthroplasty for glenohumeral arthritis: a systematic review of the literature at long-term follow-up. Int J Shoulder Surg 2013;7:110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]