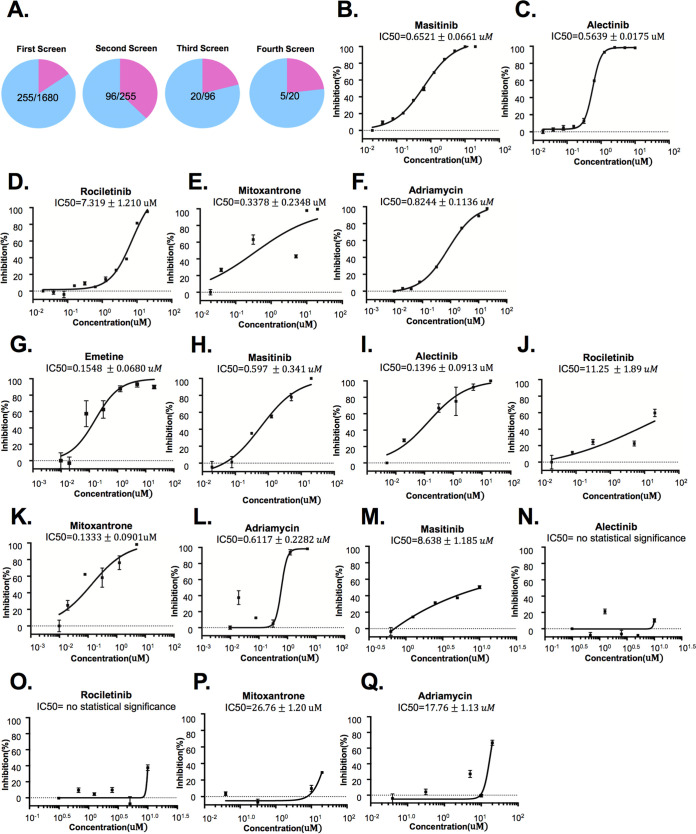
FIG 4.
Clinical-use compound library screening based on SARS-CoV-2 replicon system. (A) ps2V (12.5 ng), ps2AN (6.25 ng), ps2AC (50 ng), and ps2B (50 ng) were cotransfected into 293T cells (cell density: 6.5 × 104/cm2) seeded in a 96-well plate. After transfection for 24 to 30 h, drugs were added into cell medium for 24-h treatment before detecting luciferase activity. After the fourth round of screening, 5 hit compounds were selected for further examination. (B to F) After transfection for 24 to 30 h, the indicated drugs were added into the cell medium. The luciferase activity was detected after drug treatment for 24 h. IC50 of candidate drugs inhibiting replicon was measured using 6 to 10 different concentrations. The inhibition (%) was calculated by the formula (1 − Luceach concentration/LucDMSO) × 100%. (G to L) The IC50 of candidate drugs inhibiting authentic virus strains. The authentic SARS-CoV-2 infection assay was performed according to Materials and Methods. Drugs were added into cell medium for treatment for 24 h. Virus RNA was isolated, and RT-qPCR was performed to detect virus RNA copies. The inhibition (%) was calculated as (1 − viral RNA copieseach concentration/viral RNA copiesDMSO) × 100%. (M to Q) IRES-CMV-luciferase plasmid was transfected into 293T cells (cell density: 6.5 × 104/cm2). After transfection for 24 to 30 h, the indicated drugs were added into the cell medium. The luciferase activity was detected after drug treatment for 24 h. IC50 of the drugs was measured using different concentrations. The inhibition (%) was calculated by the formula (1 − Luceach concentration/LucDMSO) × 100%. All experimental data were analyzed using GraphPad Prism. All data are representative of at least three experiments.