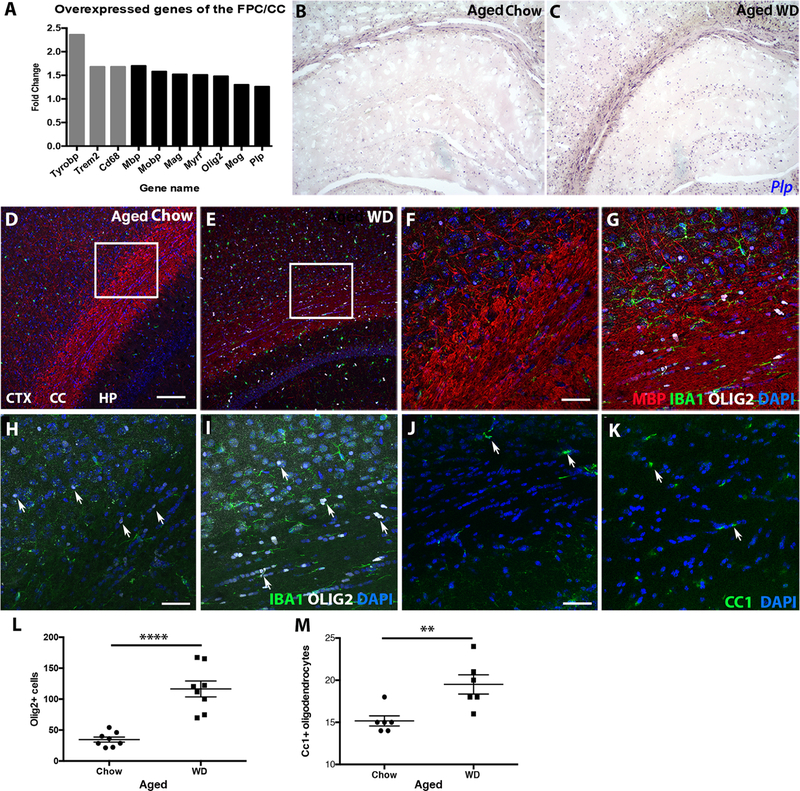
Figure 5. WD-induced white matter damage is caused by unbalanced myelin turnover.
(A) Gene expression analysis of the FPC/CC showed genes associated with phagocytosis (grey bars), myelin maintenance and oligodendrocytes (black bars) were upregulated in aged WD compared to aged chow mice. (B, C) By RNA in situ hybridization, expression of Plp, a marker of mature oligodendrocytes, was greater in the cortex, corpus callosum, and hippocampus of aged WD compared to aged chow mice. (D, E) By immunofluorescence, aged WD mice showed a significant increase in OLIG2+ oligodendrocytes in both the FPC and corpus callosum. (F, G) Higher resolution images of the areas from the inlays in E and F showed OLIG2+ cells aligned in the corpus callosum of aged WD mice. (H, I) Higher resolution images of the areas from the inlays in E and F without myelin staining showed OLIG2+ cells aligned in the corpus callosum of aged WD mice (arrows, OLIG2+ cells, white). (J, K) By immunofluorescence, aged WD mice showed a significant increase in CC1+ oligodendrocytes in the corpus callosum (arrows, CC1+ cells, green). (L) OLIG2+ cells are significantly increased in the corpus callosum in aged WD compared to aged chow mice (n=8, ****p<0.0001). (M) CC1+ cells are significantly increased in the corpus callosum in aged WD compared to aged chow mice (n=6, **p<0.01). Scale bars: D-E, 100µm; F-K, 40µm.