Fig. 1. Asymmetrical constricted conformation of the selectivity filter.
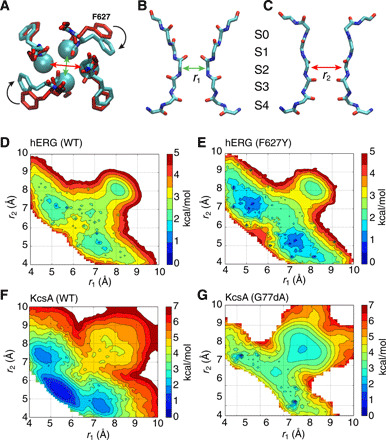
(A to C) The C2 model based on an average structure (cyan) symmetrized by swapping between two opposite subunits shown from the top view is overlaid with the cryo-EM structure (red) (A), two side views highlighting different pairs of subunits with ion-binding sites marked (B and C). (D to G) 2D-PMF of the hERG and KcsA channels reveals local free energy basins corresponding to a general asymmetrically constricted conformation. The horizontal and vertical reaction coordinates, respectively, represent the cross-subunit distance between the Cα atoms of glycine (G626 in hERG or G77 in KcsA) of diagonally opposed subunits A and C (r1), and B and D (r2). Results for WT KcsA (F) and KcsAD-ala77 (G) from a similar PMF calculation were previously reported (18).
