Figure 4.
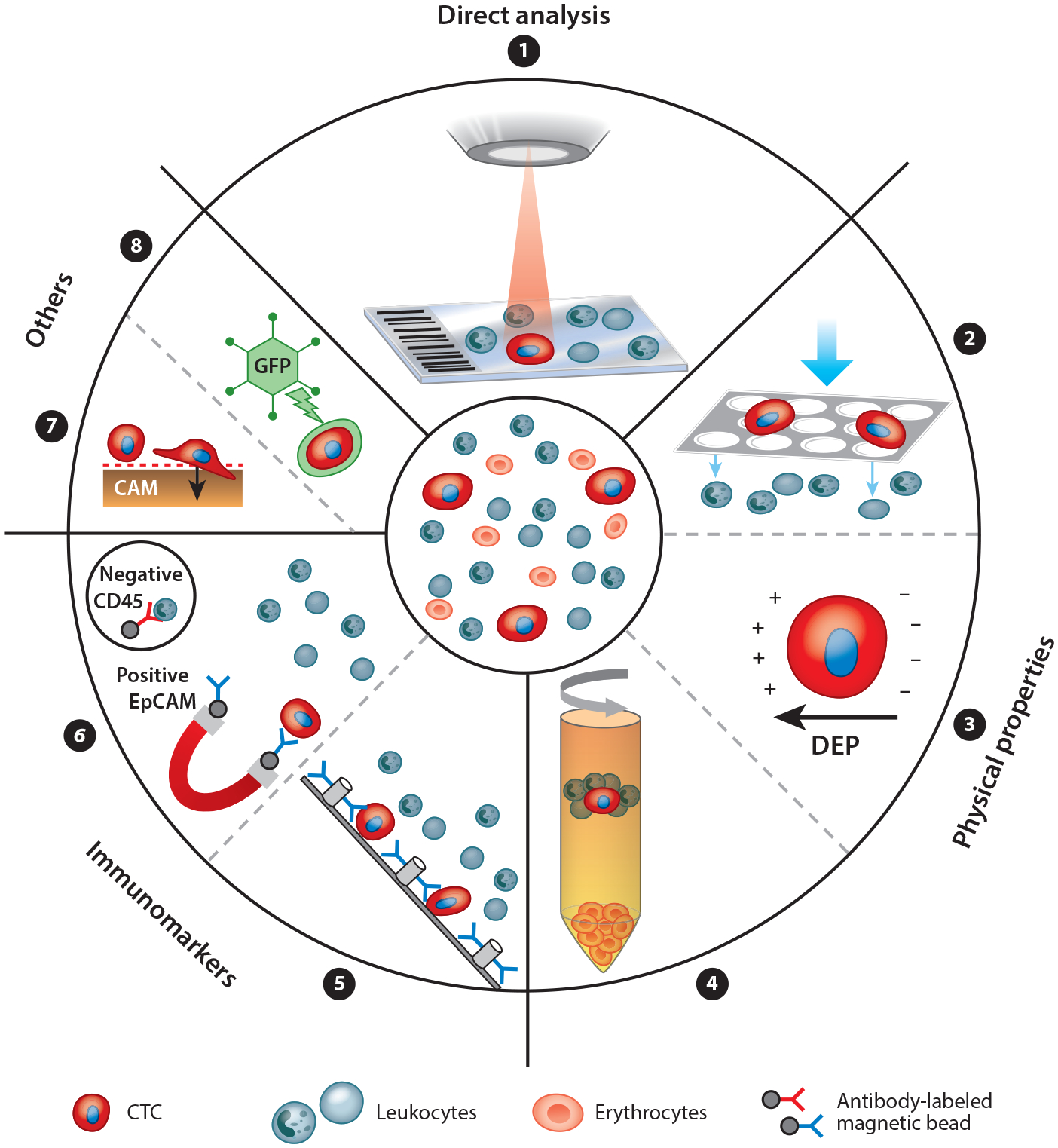
The principles of circulating tumor cell (CTC) enrichment. CTCs can be detected in many ways. A nonenrichment approach involves the direct analysis, by microscopy, of all peripheral blood mononuclear cells of a patient sample (➊). The enrichment approaches are divided into label dependent (addressing protein expression) and non–label dependent (enrichment by physical properties of CTCs) approaches. Positive or negative enrichment by physical properties (➋➌➍) is possible, for example, using filtration by size (e.g., ISET®) (➋), using dielectrophoretic properties (DEPs) (➌), or using centrifugation through a Ficoll density gradient (➍). Enrichment through immunomarker positivity (e.g., for epithelial markers like EpCAM or mesenchymal markers like N-cadherin) is a common and well developed method (➎➏). Label-dependent techniques have been optimized by many microdevices like the CTC-Chip by using antibody-labeled microposts (➎). Alternatively, negative enrichment may be performed by depletion of leukocytes using antibodies against CD45 (➏). Some other approaches on the market use specific CTC functions like protein secretion (EPISPOT) or invasive behavior on a cell adhesion matrix (CAM) (➐). DNA targets can be detected within CTCs through aptamer technology or through oncolytic green fluorescent protein (GFP)–containing, telomerase-specific adenoviruses (➑).
