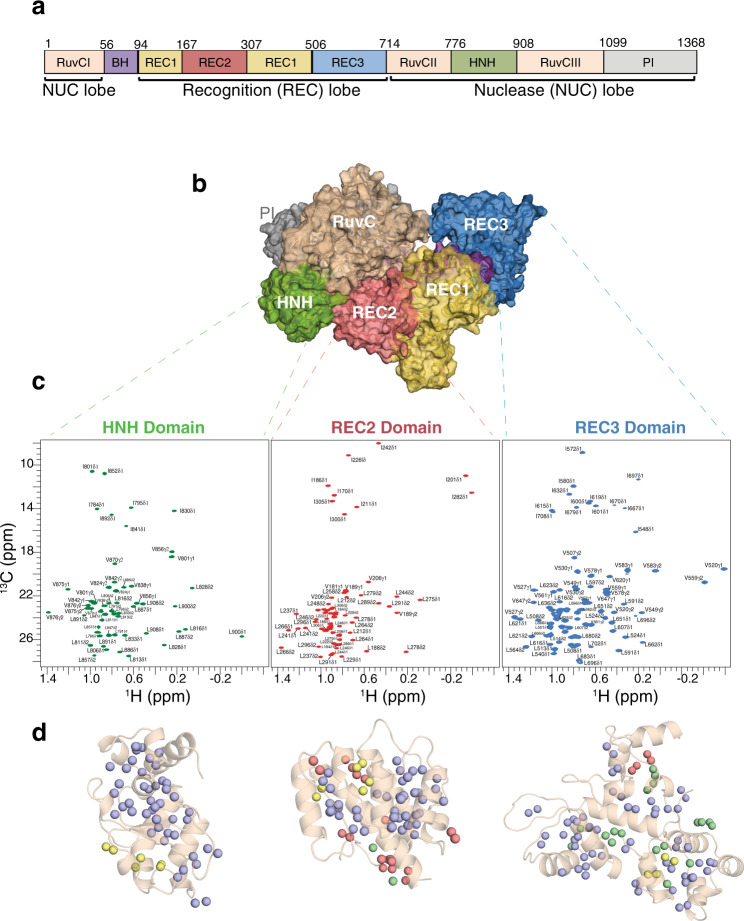
Fig. 4. Streamlined resonance assignments of Cas9 domains using MAUS.
a Domain organization of SpyCas9 composed of the recognition lobe (REC) and nuclease lobe (NUC). BH, bridge helix; PI, PAM-interacting. b Surface representation of SpyCas9 (PDB ID 4cmp) depicting the bilobed architecture. Protein domains are colored as in a. c Single domains used in the divide-and-conquer approach. 1H-13C HMQC spectra of selectively labeled HNH (green), REC2 (red), and REC3 (blue) domains at Ile δ1-13CH3; Leu, Val-13CH3/13CH3 positions, acquired at 800 MHz, 25 °C. The spectra of the individual domains of Cas9 indicate that they retain their proper fold when in isolation. d Number of valid assignment options for each residue identified by MAUS for HNH, REC2, and REC3, respectively. The colored spheres represent final valid resonance assignment options: violet (1 option), green (2 options), yellow (3 options), and red (>3 options).