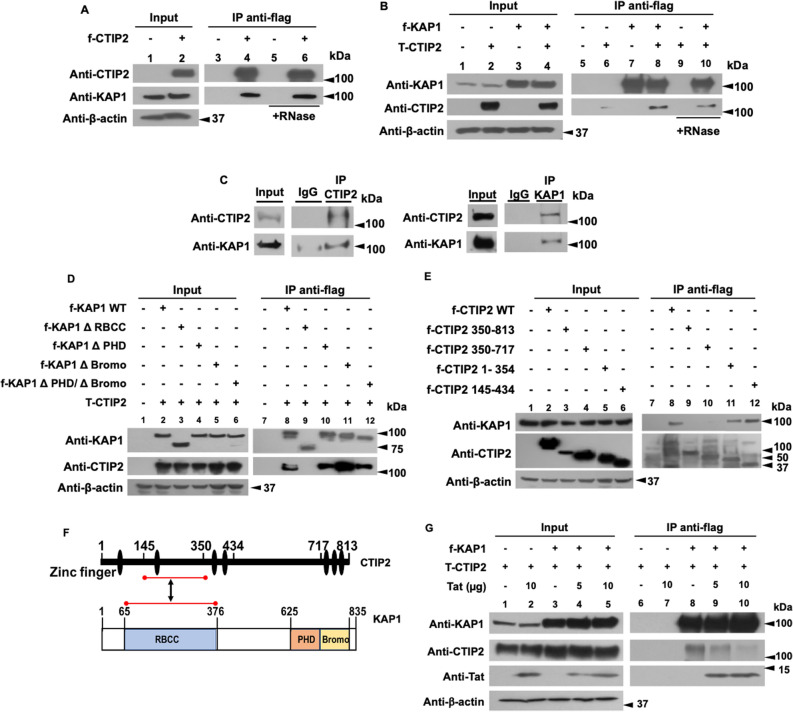
Figure 8.
CTIP2 interacts with KAP1 in an RNA independent manner and competes with Tat for KAP1 binding (A,B) HEK cells were transfected with flag-CTIP2 (A) or flag-KAP1 and Tap-CTIP2 (B). 48 h post-transfection, the nuclear protein extracts, treated or not with RNase, were subjected to immunoprecipitation with the flag antibody. The immunoprecipitated complexes were tested by Western blot for the presence of KAP1 (A) and CTIP2 (B). (C) The nuclear protein extracts of microglial cells were analyzed by Western blot for the expression of CTIP2 and KAP1 and submitted to immunoprecipitation experiments with CTIP2 and KAP1 antibodies. (D) The HEK cells were transfected with Tap-CTIP2 and one of the vectors encoding f-KAP1 WT: wild type, or KAP1 deleted from the RBCC domain (ΔRBCC), or PHD (ΔPHD), or Bromo (ΔBromo) or both PHD and Bromo domains (ΔPHD/ΔBromo), or with f-CTIP2 deletion mutants, as indicated (E). After 48 h, the nuclear protein extracts were immunoprecipitated with flag antibody. The eluted protein complexes were analyzed by Western blot for the presence of CTIP2 and KAP1. (F) The interface between CTIP2 and KAP1 structural domains are presented. (G) HEK cells were transfected with the indicated vectors in the presence of an increasing dose of Tat. After 48 h, the nuclear protein extracts were treated as in (D). The results are representative of at least two independent experiments. Full-length blots are presented in Supplementary Figure 9.