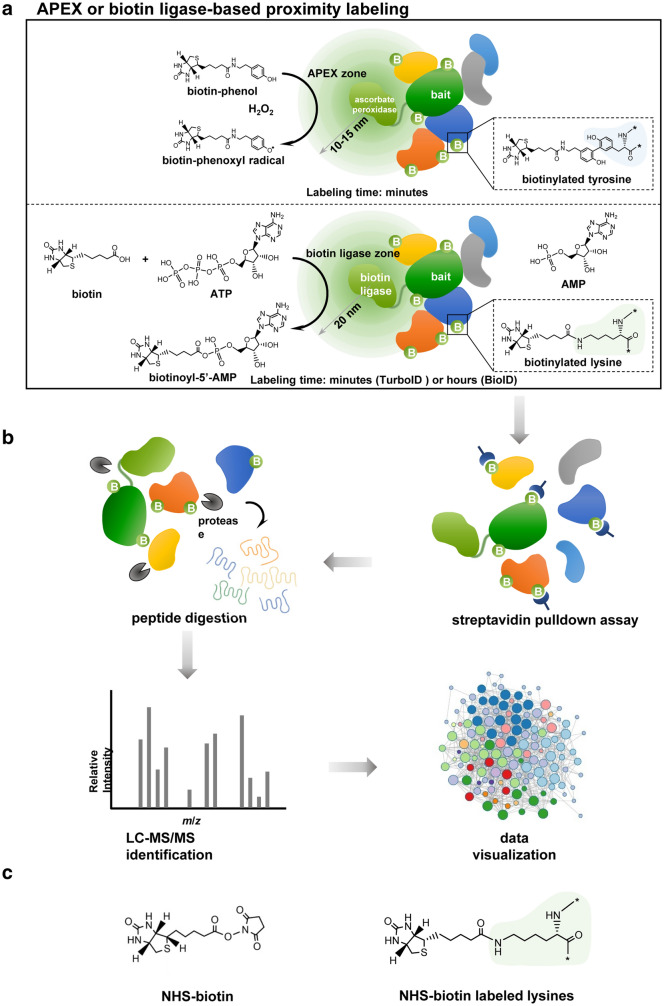
Fig. 1.
Schematic diagram of enzyme-catalytic proximity labeling approaches.
a In a standard enzyme-catalytic proximity labeling system, ascorbate peroxidase (e.g., APEX or APEX2) in the presence of H2O2 catalyzes the one-electron oxidation of biotin-phenol into a highly reactive and short-lived biotin-phenoxyl radical, which biotinylates tyrosine predominantly in nearby proteins (upper panel). In contrast biotin ligase (e.g. BioID or TurboID/miniTurbo) catalyzes the synthesis of a biotinoyl-5’-AMP intermediate from biotin and ATP and promiscuously tags lysine in nearby proteins (lower panel). These enzymes can be fused in frame to the bait protein and introduced into living cells. The biotinylation process depends on the localization of the bait protein and occurs in a proximate dependent manner. b The biotinylated proteins are first enriched and purified by streptavidin pulldown assay, then further digested into peptides and identified by quantitative LC-MS/MS. Subsequently, a specific data analysis scheme is adopted for data visualization based on the experimental purpose and design. c The chemical structure of NHS-biotin (left panel), which can biotinylate lysine in any protein without a requirement for enzymatic catalyzation (right panel)