Figure 1. Optimization of organoid culture conditions.
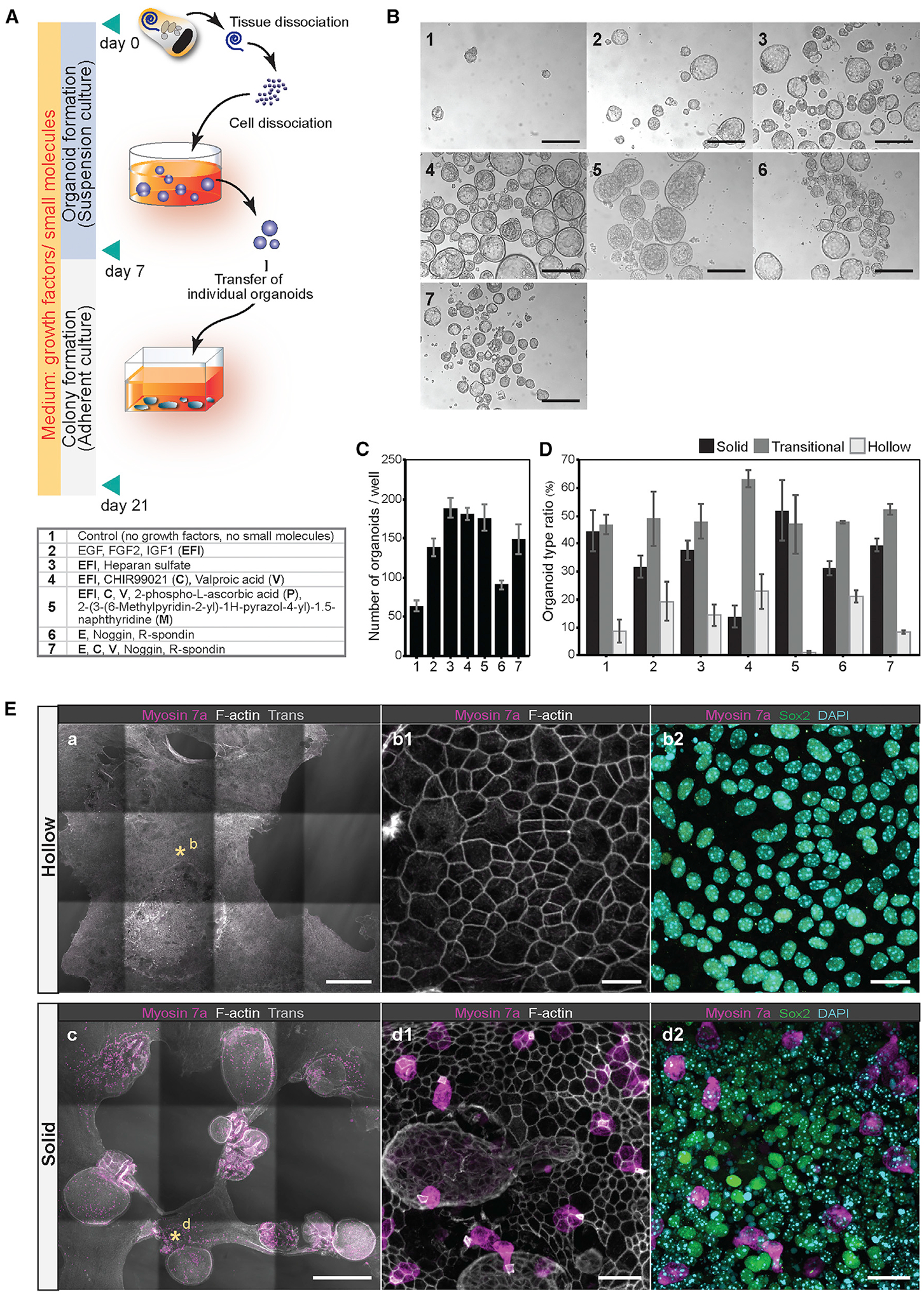
(A) Process of P2 mouse cochlear cell dissociation and suspension culture. Dissociated cells are cultured for 7 days to generate organoids, followed by 14 days of adherent culture. For comparing different media supplements, dissociated cells were plated at 50 cells/μL density in 24-well dishes (25,000 cells/well). Conditions 1–7 are listed in the table.
(B) Representative organoids that formed after 7 days in culture. Conditions 1–7 are shown. Scale bars: 200 μm.
(C and D) The number of organoids (C) and the ratio of the three organoid morphologies (D) after 7 days in culture. Shown are the means ± standard deviations (SD) of triplicate experiments.
(E) Colonies generated from organoids after 21 days in cultures supplemented with EFI_CVPM (condition 5). Myosin 7a-expressing cells were not detected in colonies grown from hollow-type organoids (a, b1, and b2). The supporting cell/otic progenitor cell marker Sox2 was detected. Myosin 7a-expressing cells were detected in colonies grown from solid-type organoids (c, d1, and d2). Asterisks in (a) and (c) highlight the regions shown magnified in (b1), (b2), (d1), and (d2). Scale bars: 500 μm (a); 20 μm (b1 and b2); 500 μm (c); and 20 μm (d1 and d2).
