Abstract
Background
The therapeutic approach to COVID-19 and healthcare system preparedness improved during 2020. We compared characteristics and outcomes of hospitalized COVID-19 patients during the first 28 days of the March and October pandemic waves in Milan, Italy.
Material and methods
A prospective, observational study enrolling adult patients hospitalized with COVID-19 pneumonia during March 7-April 4 (1st period) and October 15-November 12 (2nd period). During the 1st period hydroxychloroquine, lopinavir/ritonavir and therapeutic enoxaparin when thrombosis was confirmed were administered; systemic corticosteroids were given in case of severe pneumonia. During the 2nd period dexamethasone, methylprednisolone, remdesivir, thromboprophylaxis or anticoagulation were administered according to international recommendations. Patients with respiratory distress on oxygen masks initiated CPAP. Outcomes were: length of hospital stay, all-cause in-hospital mortality and need for intubation.
Results
We included 70 patients (75% males) during the 1st and 76 patients (51% males, p = 0.522) during the 2nd period. Prevalence of severe respiratory failure (30% vs. 12%, p = 0.006), and D-dimer >3000 FEU (34% vs. 15%, P = 0.012) were reduced during the 2nd period, while anticoagulation and corticosteroids were more frequently administered (both p < 0.01). Mortality and time to referral were also reduced (39.4% vs. 22.4%, p = 0.019 and 6 vs. 5 days, p = 0.014), while need for intubation didn't change. Hospitalization length was comparable, but the proportion of patients discharged home was higher during the 2nd period (28.2% vs. 55.4%, p = 0.001).
Conclusions
Changing treatment paradigms and early referral might have reduced mortality in COVID-19 patients. The effects of specific therapeutic regimens needs further confirmation in future clinical studies.
Keywords: COVID-19, Respiratory failure, Pneumonia, Treatment, CPAP, Mortality
Abbreviations: COVID-19, Coronavirus Disease 2019; CPAP, Continuous Positive Airway Pressure; CRP, C reactive protein; DNI, do not intubate order; ED, emergency department; FEU, fibrinogen equivalent unit; FiO2, inspired oxygen fraction; HDRU, high dependency respiratory unit; Hb, hemoglobin; IMV, invasive mechanical ventilation; LDH, lactic acid dehydrogenase; LMWH, Low Molecular Weight Heparin; PaO2, peripheral arterial oxygen partial pressure; PaCO2, peripheral arterial carbon dioxide partial pressure; PEEP, positive end expiratory pressure; SaO2, peripheral oxygen saturation; WBC, white blood cells
1. Introduction
Based on groundbreaking research, over the course of 2020 the treatment approach applied to the Coronavirus Disease 2019 (COVID-19) has constantly evolved. Moreover, a higher preparedness of the healthcare system in the identification of infected patients has reduced the time for patients’ referral to seek medical assistance. To date, however, if these changes may have influenced the outcomes of patients affected by COVID-19 pneumonia remains poorly understood.
The aim of the study was to compare the clinical characteristics and outcomes of patients hospitalized with COVID-19 pneumonia during the first four weeks of the March and October pandemic waves in the Italian region of Lombardy.
2. Material and methods
This was an observational prospective study in which all patients hospitalized in the High Dependency Respiratory Unit (HRDU) of the L. Sacco University Hospital in Milan (Italy) with community-acquired COVID-19 pneumonia from March 7th until April 4th (1st period) and from October 15th until November 12th (2nd period) were consecutively enrolled. Inclusion criteria were: i) a Severe Acute Respiratory Syndrome Coronavirus type 2 (SARS-CoV-2) infection confirmed by a nasopharyngeal swab (NFS), ii) age ≥ 18 years. Patients accessed the HDRU if satisfied at least one of the following criteria: need for FiO2 ≥40% to maintain an SpO2 ≥94%, a respiratory rate>25/min while on oxygen therapy, or the presence of respiratory distress.
Clinical outcomes were: need for invasive mechanical ventilation (IMV), length of hospital stay, HDRU and in-hospital all-cause death.
Clinical characteristics, vital signs and gas exchange parameters were collected at arrival in the emergency department (ED). According to local standard operating procedures and Italian recommendations [[1], [2], [3]], unless contra-indicated, during the 1st period patients were administered hydroxychloroquine, lopinavir/ritonavir and off-label immunomodulation with tocilizumab. Unless contra-indicated, prophylactic low molecular weight heparin (LMWH) was administered to all patients needing bed rest and at risk of deep vein thrombosis (DVT). Therapeutic LMWH was administered in case of confirmed DVT or pulmonary embolism, or with a D-dimer value > 5000 fibrinogen equivalent units (FEU). Systemic methylprednisolone was administered in patients with severe pneumonia as recommended by international guidelines on community acquired pneumonia [4]. During the 2nd period, remdesivir [5], dexamethasone 6 mg/day [6] and methylprednisolone 80 mg/day [7] were administered following evidence based data, LMWH was given to all hospitalized patients (unless contraindicated), while therapeutic LMWH dosages were used in all critical patients or with a D-dimer ≥3000 FEU [8]. During both periods, helmet Continuous Positive Airway Pressure (CPAP) was initiated in all patients with respiratory distress or with a PaO2/FiO2<250 mmHg on 100% FiO2 reservoir masks, as previously reported [[9], [10], [11]], although during the 2nd period, standard operating procedures for positive end expiratory pressure (PEEP) were modified not to exceed 10 cmH2O. Criteria for intubation and for a Do-Not-Intubate (DNI) order followed local standard operating procedures, and were discussed by the treating physician with the critical care staff, considering patients' probability of hospital and ICU survival based on clinical status, comorbidities patient's own opinion and frailty score [9,10].
The study protocol (ClinicalTrials.gov: NCT04307459) followed the amended Declaration of Helsinki (2013) and was approved by the local ethical committee (Comitato Etico Milano Area I; 17263/2020). All patients gave written informed consent to participation.
2.1. Statistical analysis
Data were reported as mean and standard deviation (SD) or median and inter-quartile range (IQR) based on distribution assessed by means of Kolmogorov-Smirnov test. Qualitative variables were reported as frequencies. Qualitative and quantitative variables were compared with the Chi-squared or Fisher exact test, Student t-test or Mann–Whitney, as appropriate depending on the parametric or non parametric distribution. Kaplan-Meier survival curves were plotted to show differences in all-cause mortality between the two pandemic periods, in the whole study sample and patients treated with CPAP, log-rank test was computed to assess the presence of any statistically significant differences. A two-tailed p-value <0.05 was considered statistically significant. The analysis was performed with “IBM SPSS Statistics for Windows”, version 23 (IBM Corp, Armonk, NY, USA).
3. Results
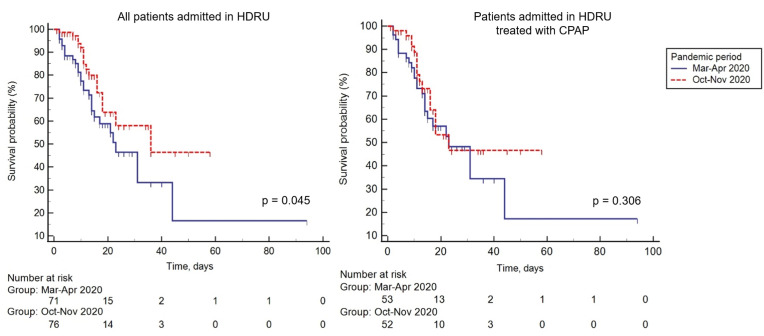
A total of 70 patients (75% males, median (IQR) age: 69 (60–80) years) were admitted during the 1st period, while 76 patients (51% males, age: 68 (55–77) years) were admitted during the 2nd period. Prevalence of asthma was significantly higher during the 2nd period (p = 0.022). During the 2nd period, patients had a lower SpO2 (95% (90–98) vs. 93% (89–97), p = 0.039) and PaCO2 (36 mmHg (32–43) vs. 32 mmHg (28–38), p < 0.001) at admittance, but the PaO2/FiO2 ratio tended to be lower (mean (SD), 249 mmHg (111) vs. 225 (116)) and the proportion of patients with severe aRF was significantly higher during he 1st period (30% vs.11.8%, p = 0.006). Except for D-dimer, that tended to be higher during the 1st period, with a significantly higher proportion of patients with a D-dimer>3000 μL/L FEU, serum were comparable between the two periods (Table 1 ). As expected, hydroxychloroquine and lopinavir/ritonavir were not used during the 2nd pandemic wave, while the proportion of patients treated with systemic corticosteroids and therapeutic doses of LMWH significantly increased (Table 1). Compared with the 1st pandemic wave, during the 2nd period the PEEP applied during CPAP therapy was significantly lower (10 cmH2O (min.5 - max.15) vs. 7.5 cmH2O (min.5 – max.10), p < 0.001) The time elapsed between symptoms’ onset and referral to the ED was shorter during the 2nd period, paralleled by a significant increase in the number of discharged patients (Table 1), and a decrease in all-cause HDRU (28.2% vs. 11.8%, p = 0.011) and in-hospital mortality (39.4% vs. 22.4%, p = 0.019) (Fig. 1 ). When limiting the analysis to patients treated with CPAP, during the 2nd period patients experienced a significantly shorter time from symptoms to ED referral and from ED admission to the potential intubation. Accordingly, all-cause in-hospital and HDRU mortality tended to be lower (Table 1, Fig. 1).
Table 1.
Clinical characteristics, serum biomarkers, in hospital treatments and outcomes of patients hospitalized with COVID-19 pneumonia.
| Apr–Mar (n = 70) | Oct–Nov (n = 76) | p-value | Treated with CPAP |
||||
|---|---|---|---|---|---|---|---|
| Mar–Apr (n = 53) | Oct–Nov (n = 52) | p-value | |||||
| Age, years | 69.5 (60–79.9) | 68 (55.2–77.0) | 0.871 | 68 (9.5) | 65 (11.6) | 0.169 | |
| Age >65 years, n (%) | 44 (62) | 45 (59) | 0.431 | 35 (66) | 30 (58) | 0.249 | |
| Males. n (%) | 53 (75) | 56 (51) | 0.522 | 41 (77) | 37 (71) | 0.307 | |
| Comorbidities, n (%) | |||||||
| Arterial hypertension | 33 (46.5) | 33 (43.4) | 0.419 | 26 (49.1) | 24 (46.2) | 0.459 | |
| Diabetes mellitus | 13 (18.3) | 23 (30.3) | 0.067 | 12 (22.6) | 16 (30.8) | 0.236 | |
| Ischaemic heart disease | 13 (18.3 | 17 (22.4) | 0.343 | 12 (22.6) | 13 (25.0) | 0.478 | |
| Heart failure | 8 (11.3) | 5 (6.6) | 0.239 | 6 (11.3) | 5 (9.6) | 0.514 | |
| Arrythmia | 11 (15.5) | 7 (9.2) | 0.182 | 9 (17.0) | 2 (3.8) | 0.028 | |
| COPD | 3 (4.2) | 8 (10.5) | 0.127 | 2 (3.8) | 4 (7.7) | 0.330 | |
| Asthma | 1 (1.4) | 8 (10.5) | 0.022 | 1 (1.9) | 8 (15.4) | 0.014 | |
| Obesity | 5 (7.0) | 9 (11.8) | 0.240 | 4 (7.5) | 9 (17.3) | 0.110 | |
| Dementia | 9 (12.7) | 3 (3.9) | 0.050 | 7 (13.2) | 0 (0) | 0.007 | |
| Psychiatric disorders | 2 (2.8) | 4 (5.3) | 0.373 | 0 (0) | 2 (3.8) | 0.243 | |
| Cancer | 7 (9.9) | 7 (9.2) | 0.578 | 1 (1.9) | 0 (0) | 0.473 | |
| Chronic kidney disease | 6 (8.5) | 6 (7.9) | 0.569 | 4 (7.5) | 5 (9.6) | 0.488 | |
| Gas exchange | |||||||
| Respiratory rate, bpm | 27 (22–31) | 28 (22–33) | 0.411 | 28 (7) | 29 (8) | 0.252 | |
| SaO2, % | 95 (90–98) | 93 (89–97) | 0.039 | 92 (13) | 90 (13) | 0.071 | |
| FiO2 | 29 (21–60) | 21 (21–37) | 0.097 | 47 (21–79) | 21 (21–58) | 0.164 | |
| pH | 7.46 (0.06) | 7.48 (0.06) | 0.110 | 7.46 (0.07) | 7.48 (0.06) | 0.207 | |
| PaO2, mmHg | 70.5 (56.0–137.0) | 65 (56.0–97.4) | 0.198 | 77 (36) | 65 (23) | 0.051 | |
| PaCO2, mmHg | 36 (32.0–43.2) | 32 (28.0–38.4) | < 0.001 | 36 (7) | 31 (6) | 0.001 | |
| PaO2/FiO2, mmHg | 225 (116) | 249 (111) | 0.208 | 196 (97) | 218 (103) | 0.247 | |
| PaO2/FiO2 | <100, n (%) | 10 (14.3) | 12 (15.8) | 0.492 | 10 (18.9) | 12 (23.1) | 0.386 |
| 100-199, n (%) | 21 (30.0) | 9 (11.8) | 0.006 | 18 (34.0) | 6 (11.5) | 0.006 | |
| 200-299, n (%) | 21 (30.0) | 28 (36.8) | 0.242 | 17 (32.1) | 22 (42.3) | 0.189 | |
| ≥300, n (%) | 18 (25.7) | 27 (35.5) | 0.135 | 8 (15.1) | 12 (23.1) | 0.214 | |
| Biochemistry | |||||||
| WBC, x109/L | 6.93 (5.21–10.57) | 7.42 (5.36–10.02) | 0.831 | 8.79 (4.88) | 7.94 (3.06) | 0.252 | |
| Hb, g/dL | 13.1 (12.5–14.6) | 13.8 (12.5–14.7) | 0.263 | 13.3 (1.7) | 13.4 (1.7) | 0.639 | |
| PLT, x109/L | 222 (101) | 216 (209) | 0.582 | 229 (108) | 226 (83) | 0.911 | |
| Lymphocytes, % | 12.6 (7.5–18.2) | 13.8 (8.4–18.0) | 0.372 | 13.2 (9.0) | 14.2 (10.8) | 0.601 | |
| Lymphocytes, x109/L | 0.89 (0.61–1.31) | 0.89 (0.63–1.17) | 0.921 | 0.96 (1.04) | 0.47 (1.28) | 0.675 | |
| D-dimer, μL/L F.E.U. | 1195 (764–5428) | 948 (598–1906) | 0.060 | 1288 (803–6063) | 921 (598–4049) | 0.046 | |
| D-dimer ≥ 1000 μL/L FEU | 33 (59) | 34 (47) | 0.128 | 29 (61.7) | 23 (44.2) | 0.062 | |
| D-dimer ≥ 3000 μL/L FEU | 19 (34) | 11 (15) | 0.012 | 18 (38.3) | 6 (11.5) | 0.002 | |
| Creatinine, mg/dL | 0.88 (0.74–1.23) | 0.89 (0.69–1.29) | 0.659 | 1.04 (1.19) | 1.19 (0.91) | 0.280 | |
| Urea, mg/dL | 47 (26–63) | 45 (31–53) | 0.357 | 56 (30) | 56 (42) | 0.987 | |
| LDH, U/L | 439 (341–563) | 363 (280–541) | 0.166 | 490 (169) | 474 (180) | 0.686 | |
| CRP, mg/L | 120 (44–169) | 90 (44–150) | 0.347 | 145 (108) | 121 (84) | 0.222 | |
| AST, U/L | 42 (30-619 | 35 (23–56) | 0.143 | 50 (28) | 50 (43) | 0.998 | |
| In-hospital treatment | |||||||
| Hydroxychloroquine, n (%) | 67 (94.4) | 0 (0) | < 0.001 | 50 (94.3) | 0 (0) | < 0.001 | |
| Ritonavir/lopinavir, n (%) | 62 (87.3) | 0 (0) | < 0.001 | 46 (86.8) | 0 (0) | < 0.001 | |
| Remdesivir, n (%) | 0 (0%) | 2 (2.7) | 0.262 | 0 (0) | 2 (3.9) | 0.238 | |
| Methylprednisolone, n (%) | 27 (38.0) | 48 (63.2) | 0.002 | 24 (45.3) | 42 (80.8) | < 0.001 | |
| Desametasone, n (%) | 0 (0) | 26 (34.2) | < 0.001 | 0 (0) | 8 (15.4) | 0.003 | |
| LMWH prophylaxis, n (%) | 52 (73.2) | 28 (36.8) | < 0.001 | 42 (79.2) | 14 (26.9) | < 0.001 | |
| LMWH therapeutic, n (%) | 9 (12.7) | 47 (61.8) | < 0.001 | 8 (15.1) | 38 (73.1) | < 0.001 | |
| CPAP, n (%) | 53 (74.6) | 52 (68.4) | 0.257 | – | – | – | |
| PEEP, cmH2O | 10 (10–10) | 7.5 (7.5–7.5) | < 0.001 | – | – | - | |
| CPAP duration, days | 7 (4–12) | 6 (4–9) | 0.212 | – | – | – | |
| Outcomes | |||||||
| Length of stay, days | 13 (6–18) | 12 (9–17) | 0.657 | 13 (7–18) | 12 (7–17) | 0.825 | |
| Symptoms to ED, days | 6 (6–18) | 5 (6–13) | 0.014 | 7.5 (5.7) | 5.5 (3.8) | 0.049 | |
| Symptoms to IMV, days | 14 (7–19) | 9 (7–14) | 0.055 | 14.2 (7.7) | 9.0 (4.7) | 0.035 | |
| ED to IMV, days | 8 (5–11) | 7 (5–7) | 0.115 | 9.9 (7.7) | 6.0 (2.2) | 0.029 | |
| Discharged, n (%) | 43 (60.6) | 60 (78.9) | 0.012 | 34 (64.2) | 41 (80.4) | 0.051 | |
| Discharged | home, n (%) | 20 (28,2) | 42 (55.4) | 0.001 | 16 (30.2) | 31 (60.8) | 0.002 |
| low intensity unit, n (%) | 23 (32.4) | 18 (23.7) | 0.209 | 18 (34.0) | 10 (19,6) | 0.076 | |
| DNI order, n (%) | 32 (45.1) | 30 (39.5) | 0.302 | 25 (47.2) | 17 (32.7) | 0.094 | |
| IMV, n (%) | 10 (14.1) | 15 (19.7) | 0.245 | 10 (18.9) | 15 (28.8) | 0.166 | |
| HDRU all-cause death, n (%) | 20 (28.2) | 9 (11.8) | 0.011 | 16 (30.2) | 9 (17.3) | 0.093 | |
| In-hospital all-cause death, n (%) | 28 (39.4) | 17 (22.4) | 0.019 | 24 (45.3) | 16 (30.8) | 0.092 | |
Continuous variables are expressed as means (standard deviation) or medians (inter-quartile range) depending on data distribution, while quantitative variables are expressed as percentages. Positive end expiratory pressure is expressed as median and range. The χ2 test, the T student test for independent variables and the Mann-Whitney U test were applied to compare groups, as appropriate. Statistically significant comparisons are in bold.
CPAP: continuous positive airway pressure; CRP: C reactive protein; DNI: do not intubate order; ED: emergency department; FEU: fibrinogen equivalent unit; FiO2: inspired oxygen fraction; Hb: hemoglobin; IMV: invasive mechanical ventilation; LDH: lactic acid dehydrogenase; LMWH: low molecular weight heparin; PaO2: peripheral arterial oxygen partial pressure; PaCO2: peripheral arterial carbon dioxide partial pressure, PEEP: positive end expiratory pressure; HDRU: high dependency respiratory unit; SaO2: peripheral oxygen saturation; WBC: white blood cells.
Fig. 1.
Mortality during the 1st and 2nd pandemic wave.
Survival curves in patients admitted to the HDRU during the March–
April (blue line) and October–
November 2020 (red dotted line) pandemic periods in the whole cohort (left panel) and in patients treated with CPAP (right panel). HDRU: high dependency respiratory unit; CPAP: continuous positive airway pressure. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
In this prospective observational study we showed that, during the second pandemic wave, in-hospital and HDRU mortality for patients admitted with COVID-19 pneumonia was significantly reduced compared with the first pandemic period. The time from symptoms onset to the ED referral was shortened, enabling earlier access to IMV or CPAP for critical patients, prompt exposure to supportive care and, in general, a lower prevalence of patients with severe respiratory failure at admission. The availability of important results regarding randomized clinical trials and observational studies caused a progressive change in the treatment paradigm in patients with COVID-19 pneumonia. Differently to what was reported in other studies performed in Japan and China [14,15], we did not observe a younger population during the second wave, and, except for asthma, the prevalence of comorbidities, especially cardiovascular ones, were comparable. We thus hypothesize that a higher social awareness, a better preparedness of COVID-19 hub hospitals and a reduction in time to diagnosis due to rapid NFS processing [15] during the 2nd pandemic wave resulted in a faster patients’ referral to the emergency department in case of suspected SARS-CoV-2 infection and worsening symptoms, anticipating clinical monitoring and the management of disease-related complications. Less advanced disease stages, a lower number of patients with severe aRF at admission and an increased proportion of patients treated with anticoagulant therapy may have thus contributed to decreased mortality [14,15]. In fact, PaO2/FiO2<200 mmHg [10] and D-dimer >2.000 FEU [12] were demonstrated to be independent risk factors for adverse outcomes, while the increased use of systemic corticosteroids [6,7,14] and anticoagulation [13,14] showed a reduction in mortality in patients with severe COVID-19 pneumonia.
The major limitations of the present study are represented by the single center design and the limited size of the cohort.
5. Conclusions
Changes in treatment paradigms, a higher social disease awareness and a better preparedness of the healthcare system might have reduced mortality in patients hospitalized with COVID-19 pneumonia during two consecutive pandemic waves. The effect of specific therapeutic regimens (e.g. early application of anticoagulation or corticosteroids) on clinical outcomes needs further confirmation in future large clinical studies.
Availability of data and materials
PS had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis and had final responsibility for the decision to submit for publication. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Dejan Radovanovic: Conceptualization, Methodology, Investigation, Data curation, Writing - original draft. Stefano Pini: Investigation, Data curation, Writing - review & editing. Elisa Franceschi: Investigation, Data curation, Writing - review & editing. Marica Pecis: Investigation, Data curation, Writing - review & editing. Andrea Airoldi: Investigation, Data curation, Writing - review & editing. Maurizio Rizzi: Investigation, Data curation, Writing - review & editing. Pierachille Santus: Conceptualization, Methodology, Investigation, Data curation, Writing - original draft.
Declaration of competing interest
None.
Acknowledgments
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmed.2021.106323.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Società Italiana di Malattie Infettive e Tropicali S.I.M.I.T. Sezione Regione Lombardia. Vademecum per la cura delle persone con malattia da COVID-19. 2.0 ed. 2020. http://www.simit.org/medias/1569-covid19-vademecum-13-03-202.pdf [Google Scholar]
- 2.Vitacca M., Nava S., Santus P., Harari S. Early consensus management for non-ICU ARF SARS-CoV-2 emergency in Italy: from ward to trenches. Eur. Respir. J. 2020;55:2000632. doi: 10.1183/13993003.00632-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassetti M., Giacobbe D.R., Aliberti S., Barisione E., Centanni S., De Rosa F.G., et al. Italian society of anti-infective therapy (SITA) and the Italian society of pulmonology (SIP). Balancing evidence and frontline experience in the early phases of the COVID-19 pandemic: current position of the Italian society of anti-infective therapy (SITA) and the Italian society of pulmonology (SIP) Clin. Microbiol. Infect. 2020;26(7):880–894. doi: 10.1016/j.cmi.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metlay J.P., Waterer G.W., Long A.C., Anzueto A., Brozek J., Crothers K., et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am. J. Respir. Crit. Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. ACTT-1 Study Group Members Remdesivir for the treatment of covid-19 - final report. N. Engl. J. Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Recovery Collaborative Group. Horby P, Lim WS. Dexamethasone in hospitalized patients with Covid-19—preliminary report. [published online July 17, 2020] N. Engl. J. Med.. doi: 10.1056/NEJMoa2021436.
- 7.Salton F., Confalonieri P., Meduri G.U., Santus P., Harari S., Scala R., et al. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. Open Forum Infect. Dis. 2020;7(10) doi: 10.1093/ofid/ofaa421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spyropoulos A.C., Levy J.H., Ageno W., Connors J.M., Hunt B.J., Iba T., et al. Subcommittee on perioperative, critical care thrombosis, haemostasis of the scientific, standardization committee of the international society on thrombosis and haemostasis. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID‐19. J. Thromb. Haemostasis. 2020;18(8):1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aliberti S., Radovanovic D., Billi F., Sotgiu G., Costanzo M., Pilocane T., et al. Helmet CPAP treatment in patients with COVID-19 pneumonia: a multicentre cohort study. Eur. Respir. J. 2020;56(4) doi: 10.1183/13993003.01935-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santus P., Radovanovic D., Saderi L., Marino P., Cogliati C., De Filippis G., et al. Severity of respiratory failure at admission and in-hospital mortality in patients with COVID-19: a prospective observational multicentre study. BMJ Open. 2020;10(10) doi: 10.1136/bmjopen-2020-043651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radovanovic D., Rizzi M., Pini S., Saad M., Chiumello D.A., Santus P. Helmet CPAP to treat Acute hypoxemic respiratory failure in patients with COVID-19: a management strategy proposal. J. Clin. Med. 2020;9(4):1191. doi: 10.3390/jcm9041191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao Y., Cao J., Wang Q., Shi Q., Liu K., Luo Z., et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J. Intens. Care. 2020;8:49. doi: 10.1186/s40560-020-00466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ionescu F., Jaiyesimi I., Petrescu I., Lawler P.R., Castillo E., Munoz‐Maldonado Y., et al. Association of anticoagulation dose and survival in hospitalized COVID-19 patients: a retrospective propensity score weighted analysis. Eur. J. Haematol. 2020 doi: 10.1111/ejh.13533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saito S., Asai Y., Matsunaga N., Hayakawa K., Terada M., Ohtsu H., et al. First and second COVID-19 waves in Japan: a comparison of disease severity and characteristics. J. Infect. 2020 Nov 2;S0163–4453(20):30693. doi: 10.1016/j.jinf.2020.10.033. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan G., Yang Z., Lin Q., Zhao S., Yang L., He D. Decreased case fatality rate of COVID-19 in the second wave: a study in 53 countries or regions. Transbound Emerg. Dis. 2020 doi: 10.1111/tbed.13819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
PS had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis and had final responsibility for the decision to submit for publication. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.