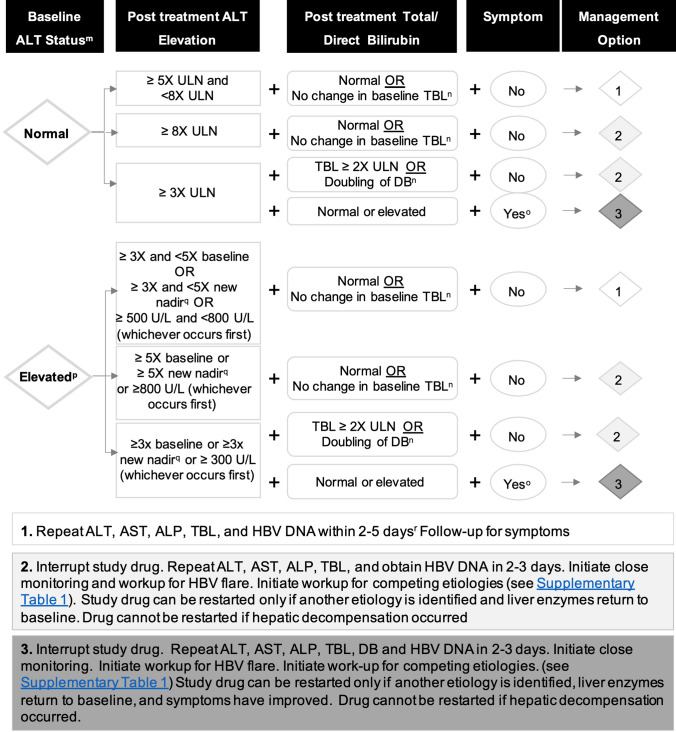
Fig. 4.
Algorithm for monitoring and management of potential DILI signals in phase II and III clinical trials for new agents to treat HBV in naïve or non-nucleos(t)ide-suppressed patients with normal or elevated baseline ALT. mBaseline ALT is derived from an average of two pretreatment ALT measurements 2 weeks apart. nFor patients with Gilbert’s syndrome or hemolysis. oSymptoms may be liver related (e.g., severe fatigue, nausea, vomiting, right upper quadrant pain) or immunologic reaction (e.g., rash, > 5% eosinophilia). pElevated baseline is defined as ALT ≥1.5 × ULN. qIn patients with a sizable stable early decrease in ALT during treatment (> 50% of baseline value), a new baseline, corresponding to the ALT nadir, should be established on an individual basis for subsequent determination of a DILI signal. rThe specific interval between the tests should be determined based on the patient’s clinical condition. ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, DB direct bilirubin, TBL total bilirubin, ULN upper limit of normal