A key survival decision, in the environment or the host, is whether to emigrate or aggregate. In bacteria, c-di-GMP signaling almost universally influences solutions to this dilemma.
KEYWORDS: swarming, c-di-GMP, GGDEF domain, EAL domain, TPR domain, RflP/YdiV
ABSTRACT
Vibrio parahaemolyticus rapidly colonizes surfaces using swarming motility. Surface contact induces the surface-sensing regulon, including lateral flagellar genes, spurring dramatic shifts in physiology and behavior. The bacterium can also adopt a sessile, surface-associated lifestyle and form robust biofilms. These alternate colonization strategies are influenced reciprocally by the second messenger c-di-GMP. Although V. parahaemolyticus possesses 43 predicted proteins with the c-di-GMP-forming GGDEF domain, none have been previously been identified as contributors to surface colonization. We sought to explore this knowledge gap by using a suppressor transposon screen to restore the swarming motility of a nonswarming, high-c-di-GMP strain. Two diguanylate cyclases, ScrJ and ScrL, each containing tetratricopeptide repeat-coupled GGDEF domains, were demonstrated to contribute additively to swarming gene repression. Both proteins required an intact catalytic motif to regulate. Another suppressor mapped in lafV, the last gene in a lateral flagellar operon. Containing a degenerate phosphodiesterase (EAL) domain, LafV repressed transcription of multiple genes in the surface sensing regulon; its repressive activity required LafK, the primary swarming regulator. Mutation of the signature EAL motif had little effect on LafV’s repressive activity, suggesting that LafV belongs to the subclass of EAL-type proteins that are regulatory but not enzymatic. Consistent with these activities and their predicted effects on c-di-GMP, scrJ and scrL but not lafV, mutants affected the transcription of the c-di-GMP-responsive biofilm reporter cpsA::lacZ. Our results expand the knowledge of the V. parahaemolyticus GGDEF/EAL repertoire and its roles in this surface colonization regulatory network.
IMPORTANCE A key survival decision, in the environment or the host, is whether to emigrate or aggregate. In bacteria, c-di-GMP signaling almost universally influences solutions to this dilemma. In V. parahaemolyticus, c-di-GMP reciprocally regulates swarming and sticking (i.e., biofilm formation) programs of surface colonization. Key c-di-GMP-degrading phosphodiesterases responsive to quorum and nutritional signals have been previously identified. c-di-GMP binding transcription factors programming biofilm development have been studied. Here, we further develop the blueprint of the c-di-GMP network by identifying new participants involved in dictating the complex decision of whether to swarm or stay. These include diguanylate cyclases with tetratricopeptide domains and a degenerate EAL protein that, analogously to the negative flagellar regulator RflP/YdiV of enteric bacteria, serves to regulate swarming.
INTRODUCTION
Rapid adaptation to fluctuating environmental conditions is critical for bacterial survival and is necessary for many host-microbe and environmental interactions. The timing and magnitude of such adaptive responses are often controlled by the intracellular second messenger molecule bis-(3′-5′)-cyclic dimeric GMP (c-di-GMP) (1, 2). Diverse bacteria contain dozens of enzymes that adjust c-di-GMP levels through synthetic, degradative, or hybrid functions, and these activities often depend on some internal or external stimulus. These enzymes are characterized by catalytic domains, which are named for highly conserved amino acid residues, responsible for formation (GGDEF) (3) or degradation (EAL) (4) of c-di-GMP. The widespread networks dedicated to modulating behaviors under the auspices of c-di-GMP underscore the importance of this regulatory mechanism among bacteria. Moreover, the breadth of the sensing capacity of these enzymes, their potential for signal integration to control c-di-GMP levels, and the scope of the output regulatory circuits provide important clues about general and unique survival strategies.
The marine bacterium and human pathogen Vibrio parahaemolyticus uses c-di-GMP to direct its surface colonization regulatory (Scr) program (Fig. 1), which controls the transition between biofilm formation and swarming motility (5–7). Surface contact invokes the surface-sensing regulon and results in swarmer cell differentiation. Hallmark characteristics of the swarmer cell include cellular elongation, lateral flagellar synthesis, virulence gene expression, and rapid locomotion over surfaces and through viscous environments (5, 8). Low levels of the second messenger c-di-GMP favor this colonization program (5, 7). Conversely, surface colonization under elevated c-di-GMP levels results in robust biofilm formation, which depends on the synthesis of various cell-associated and extracellular molecules (including capsular polysaccharide, the Mfp matrix protein, and O-antigen modification) while repressing the surface-sensing regulon (7, 9–11). Thus, c-di-GMP dictates the opposite strategies for colonizing surfaces. Two Scr enzymes have been described that affect the transition between swarming and sticking. ScrC functions as a quorum-controlled phosphodiesterase (PDE) to degrade c-di-GMP (7, 10, 12). Being a bifunctional GGDEF-EAL enzyme, its activity is modulated by ScrB (a periplasmic binding protein) and ScrA (a pyridoxal phosphate-dependent enzyme that produces the S signal autoinducer). At a high cell density, the phosphodiesterase activity of ScrC is stimulated to promote swarming (12). ScrC appears to be a major Scr PDE: deletion of the scrABC locus increases cellular c-di-GMP levels and biofilm formation while inhibiting swarming (7, 10). Another GGDEF-EAL-type protein, ScrG, also affects swarming and cell surface morphology: its sticky, swarm-defective mutant phenotype is most apparent under low-sodium growth conditions (13). Isopropyl-β-d-1-thiogalactopyranoside (IPTG)-inducible, ectopic expression of scrG+ decreases c-di-GMP levels, and this activity is eliminated upon the deletion of the EAL domain (13). Combining scrG and scrC mutations produces cumulative effects compared to single mutant phenotypes, depressing swarming motility, lateral flagellar gene expression, and lateral flagellin production and enhancing crinkly colony morphology (13). Thus, these two proteins appear to impinge upon a common c-di-GMP pool that modulates the Scr program.
FIG 1.
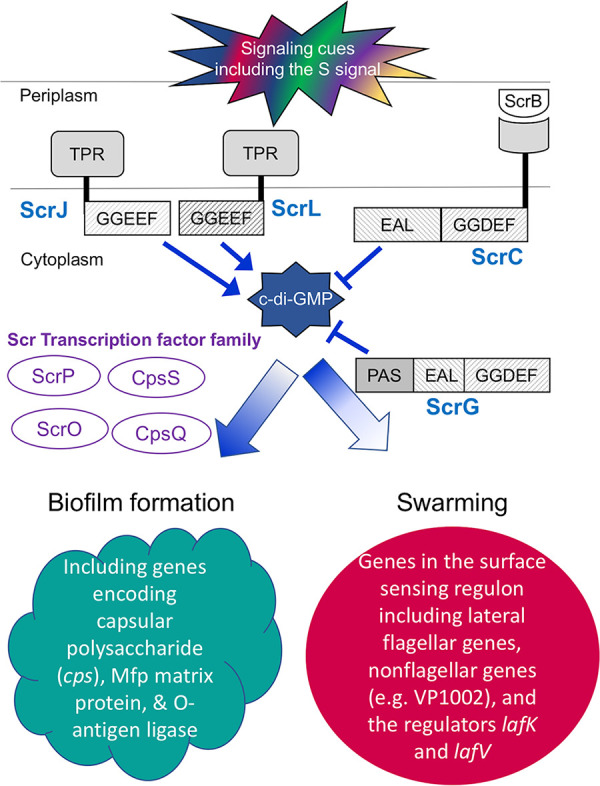
The surface colonization regulatory (Scr) network of V. parahaemolyticus. During growth on a surface, signaling via the second messenger c-di-GMP underpins regulatory decisions determining the biofilm or swarming lifestyle. High c-di-GMP levels promote biofilm development and repress the surface-sensing regulon. Diverse environmental and social cues (including the S signal autoinducer) control the activities of an array of sensory enzymes capable of modulating the level of c-di-GMP. Although they each contain catalytically active GGDEF and EAL domains capable of forming or degrading c-di-GMP, respectively, ScrC and ScrG serve physiologically as key swarming-promoting phosphodiesterases: strains lacking either protein show diminished swarming and enhanced biofilm production. Four c-di-GMP binding transcription factors, the Scr transcription factor family (“Scr TFs”), participate in controlling biofilm development by regulating cell surface genes encoding molecules such as capsular polysaccharide, Mfp matrix protein, and O-antigen ligase. The swarming lifestyle results from the induction of the surface-sensing regulon, which includes lateral flagellar and nonflagellar genes, e.g., VP1002 encoding a peptidase M60 domain-containing protein. The regulon is hierarchically expressed in three classes. Near the top of the hierarchy is the σ54-dependent regulator LafK. In this work, we describe two new GGDEF-type sensory enzymes, ScrJ and ScrL, that are members of this network. In addition, we discover a new EAL-type regulator, LafV, that modulates the surface-sensing regulon, although it does not appear to have catalytic activity.
Until now, no diguanylate cyclase (DGC) has been implicated as a primary c-di-GMP producer contributing to the Scr program. It is also unclear how c-di-GMP at high levels is itself linked to swarming gene repression. We sought to answer these questions by performing a transposon-mediated suppressor screen for swarming restoration in a nonswarming, high-c-di-GMP strain. Through our investigations, we have discovered three new genes encoding previously unknown contributors in the Scr network that negatively influence swarming. Two of these genes, scrJ (VPA1115) and scrL (VPA1069), encode tetratricopeptide repeat (TPR)-coupled GGDEF proteins, and the third, lafV (VPA1547), is the terminal gene of the lateral flagellum-associated fliM operon and paradoxically encodes an EAL domain. Here, we describe the activities of these three new regulators and their influence on swarming in V. parahaemolyticus. These findings further reveal the complexity governing the regulation of a surface colonization program vital to success in the environment and the host.
RESULTS
V. parahaemolyticus is unable to swarm under high-c-di-GMP conditions in the absence of the Scr transcription factor family.
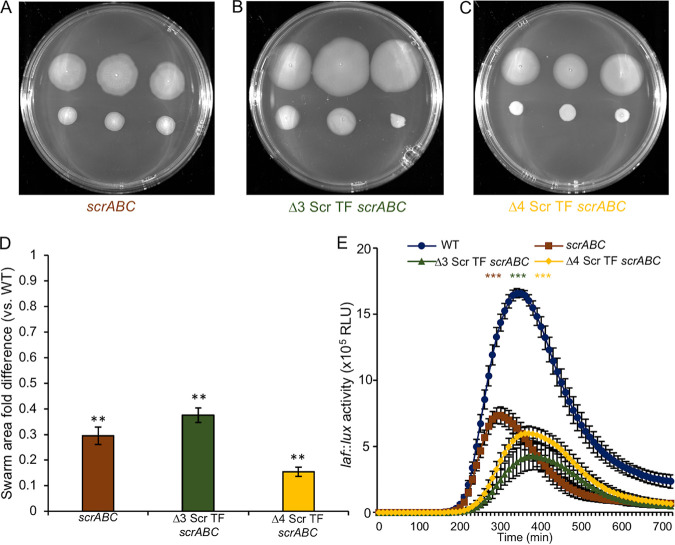
We have previously examined regulators of biofilm development. These include a family of four homologous c-di-GMP binding transcription factors (TFs) (CpsQ, CpsS, ScrO, and ScrP) that comprise the Scr transcription factor family (11, 14). The reciprocal nature of biofilm gene activation coupled with swarming gene repression suggested that at least one of these regulators of biofilm formation might also be responsible for repressing swarming genes as c-di-GMP levels rise. This hypothesis was further supported by the observation that the low-level overexpression of each of the four transcription factors (called the “Scr TFs” for their role in surface colonization regulation) reduced the activity of a luminous lateral flagellar gene reporter (called “laf::lux”) in the flgE gene, which encodes the lateral flagellar hook protein (see Fig. S1 in the supplemental material). Three of the four (CpsQ, CpsS, and ScrO) demonstrated 5- to 10-fold repressive effects, and these were diminished upon introducing an Arg-to-Ala substitution in the c-di-GMP binding pocket of each Scr TF. In fact, the alanine substitution mutation altering CpsS reversed its activity, causing laf activation. These substitution mutations were previously shown to impair c-di-GMP binding (11,14). ScrP’s repressive effect on laf gene expression was more modest (reducing activity ∼1.8-fold) and was independent of c-di-GMP binding. These data suggested that each of the Scr TFs might exert some negative effect on laf gene expression, and functional redundancy might explain why in previous studies we have been unable to identify a single master regulator linking high c-di-GMP levels to swarming repression. This potential scenario was tested by introducing Scr TF mutations into an scrABC strain, which has high c-di-GMP levels and is swarming impaired (10, 14) (Fig. 2A and D). However, strains lacking cpsQ, cpsS, and scrO (Δ3 Scr TF scrABC in Fig. 2B and D) or all four Scr TFs (Δ4 Scr TF scrABC in Fig. 2C and D) remained inhibited for swarming and repressed for laf gene expression (Fig. 2E). Figure 2 indicates subtle differences in swarm colony morphology and laf gene expression profiles between the Δ3 Scr TF scrABC, Δ4 Scr TF scrABC, and scrABC strains, suggesting some role for these transcription factors in laf regulation, albeit the roles could be direct effects on transcription or indirect, e.g., by influencing the available c-di-GMP pool as a result of eliminating known partners for c-di-GMP binding. Nevertheless, eliminating these four c-di-GMP binding regulators was insufficient to restore swarming or laf gene expression.
FIG 2.
Deleting three (scrO, cpsQ, and cpsS) or four (scrO, cpsQ, cpsS, and scrP) of the c-di-GMP binding Scr transcription factors (TFs) fails to restore swarming motility when the c-di-GMP level is elevated. (A to C) Three single colonies of the WT (LM4476) (top) and three colonies, as indicated, of the scrABC (LM11253) (A), Δ3 Scr TF scrABC (LM11427) (B), or Δ4 Scr TF scrABC (LM11430) (C) strain (bottom). (D) Quantification of ratios of swarm areas of the mutants to the WT in swarm assays performed as described above for panels A to C. The average swarm area ratio of the mutant versus the wild type was calculated for each plate, i.e., three swarming colonies of each mutant and the wild type grown on the same plate. Bars represent the averages of the ratios from at least three plates; error bars denote the standard errors. Pairwise comparisons were performed using Student’s t test (**, P < 0.01). (E) Lateral flagellar (laf::lux) gene expression during surface growth of the WT (LM1017 parental strain with flgE::lux), scrABC (LM11524), Δ3 Scr TF scrABC (LM11285), and Δ4 Scr TF scrABC (LM11525) strains. For each mutant, pairwise comparisons of maximum relative luminescence units (RLU) were performed against the WT, and statistical significance is color coordinated (***, P < 0.001).
Restoring swarming in a nonswarming strain via Tn5lux mutagenesis.
These data suggest the existence of mechanisms other than the Scr TFs linking swarming repression to high c-di-GMP levels. To further probe c-di-GMP-mediated swarming repression, we performed Tn5lux mutagenesis using our Δ3 Scr TF (ΔcpsSQ ΔscrMNO) scrABC and Δ4 Scr TF (ΔcpsSQ ΔscrMNO ΔscrP) scrABC mutant strains LM11427 and LM11430, respectively, as the parental recipients. The genes encoding cpsS and cpsQ are physically linked although in separate transcriptional units (14); therefore, a single deletion was used to eliminate both genes. scrO is found in the scrMNO operon; the operon was deleted to eliminate its entire potential role. While other negative swarming regulators have been studied in the past (7, 15–19), we reasoned that a suppressor screen selecting positively for increased swarming motility under conditions of high c-di-GMP levels (i.e., the scrABC background) could identify new participants in swarming regulation. Using LM11427 and LM11430, we performed 50 independent mutagenesis experiments (25 in each strain background), screened over 15,000 clones, and obtained 35 unique mutants with insertions in 22 genes and one intergenic region (Table S2). These mutants swarmed more proficiently than the parental strain. Within this mutant set were insertions in genes with links to factors potentially important for swarming gene activation, including genes that could influence nutritional signaling (VP1137, VP1957, VP2083, and VPA0585). Other mutants contained insertions in genes whose effects on swarming were not obvious. However, nine mutants contained insertions in three genes coding for two putative DGCs (VPA1069 and VPA1115) and one putative PDE (VPA1547) whose roles seemed directly linked to c-di-GMP levels in the cell. We therefore chose to initially focus on these three genes, which we have named scrJ (VPA1115), scrL (VPA1069), and lafV (VPA1547). Future work will investigate the other insertions.
ScrJ is a diguanylate cyclase that negatively affects swarming.
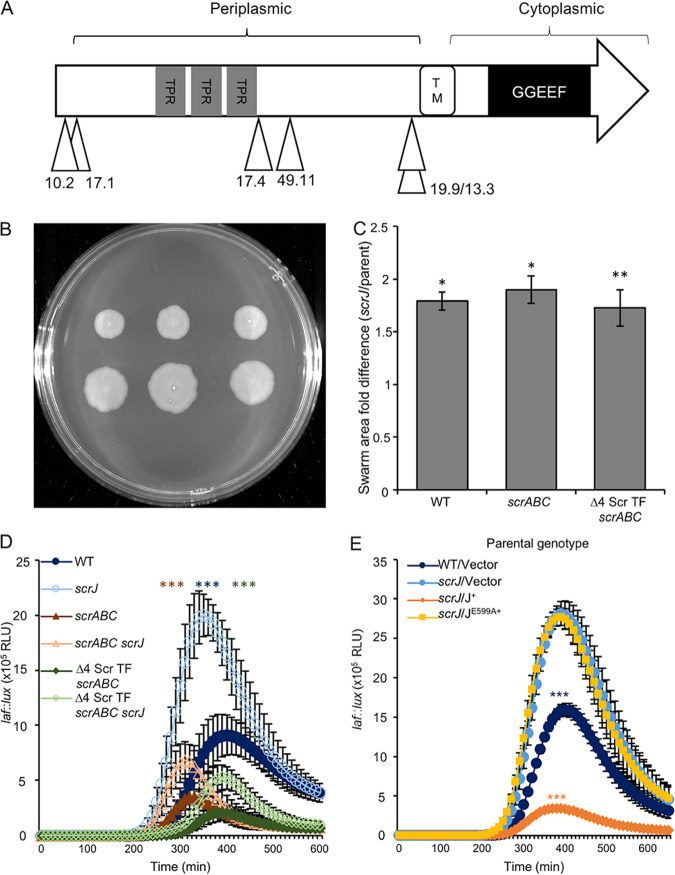
scrJ was the most frequently identified gene affecting swarming in our transposon mutant screen, appearing six times with five unique insertion sites in both strain backgrounds (Fig. 3A; Table S2). The gene (VPA1115) codes for a predicted membrane-associated DGC (78.7 kDa). The DGC’s N terminus contains a predicted periplasmic domain containing three tetratricopeptide repeat (TPR) domains; the predicted cytoplasmic C terminus contains a DGC domain (Fig. 3A). To reconstruct the mutant, we introduced an scrJ deletion into a Δ4 Scr TF ΔscrABC mutant (strain LM11985). The swarming phenotype of our original transposon isolates was recapitulated (Fig. 3B). The derepressive effect of deleting scrJ on swarming was also observed in the wild-type (WT) and scrABC strains (Fig. S2). The introduction of an scrJ deletion into any of these three backgrounds produced swarms with areas >1.5-fold larger than those of strains with a functional scrJ allele (Fig. 3C). With respect to the effects on laf gene expression, the introduction of the scrJ mutation increased laf gene expression in all strains (Fig. 3D).
FIG 3.
ScrJ is a diguanylate cyclase that negatively affects swarming. (A) Predicted domain organization of ScrJ (78.6 kDa). Gray boxes indicate three putative tetratricopeptide repeat (TPR) domains. The white box denotes a putative transmembrane helix (TM), and the black box represents the predicted GGDEF domain with the GGEEF catalytic motif. Triangles depict the sites of Tn5lux insertion obtained by mutagenesis with their corresponding strain numbers. (B) Three single swarming colonies from the Δ4 Scr TF scrABC (LM11985) (top) and Δ4 Scr TF scrABC scrJ (LM11991) (bottom) strains. (C) Quantification of the ratios of swarm areas of scrJ mutants compared to the parent, scrJ (LM11989)/WT (LM4476), scrABC scrJ (LM11990)/scrABC (LM11984), and Δ4 Scr TF scrABC scrJ (LM11991)/Δ4 Scr TF scrABC (LM11985). Bars represent the average ratios derived from at least three plates inoculated similarly to the ones in panel B. Error bars denote standard errors, and pairwise comparisons were performed using Student’s t tests (*, P < 0.05). (D and E) Lateral flagellar (laf::lux) gene expression during surface growth (n = 6). In panel D, pairwise comparisons of maximum RLU between scrJ mutants and their isogenic scrJ+ parent (e.g., LM1017 versus LM11986) were performed using Student’s t test, and statistical significance is color coordinated (**, P < 0.01; ***, P < 0.001). Strains used in panel D were the WT (LM1017 parental strain with flgE::lux), scrJ (LM11986), scrABC (LM11524), scrABC scrJ (LM11987), Δ4 Scr TF scrABC (LM11525), and Δ4 Scr TF scrABC scrJ (LM11988) strains, and strains used in panel E were the WT/Vector (LM12034), scrJ/Vector (LM12035), scrJ/J (LM12036), and scrJ/JE599A (LM12037) strains, where J indicates the scrJ allele used in trans. Strains in panel E were grown with 1 mM IPTG (n = 6). Pairwise comparisons between maximum RLU were performed against LM12035 using Student’s t test (***, P < 0.001).
To assess ScrJ’s predicted diguanylate cyclase activity, we altered its highly conserved GG(D/E)EF signature domain by introducing an E599A substitution into ScrJ’s catalytic motif (i.e., changing the signature from GGEEF to GGAEF). Analogous mutations that impair catalytic function have been introduced into many proteins with the orthologous GGDEF domain, including WspR from Pseudomonas aeruginosa (20) and HmsD (formerly Y3730) from Yersinia pestis (21). In order to assess protein production and stability, we cloned wild-type scrJ and scrJE599A into an IPTG-inducible expression plasmid with a C-terminal green fluorescent protein (GFP) tag. The constructs, along with the vector control, were introduced into an scrJ laf::lux reporter strain (LM11986). Strains ectopically expressing scrJ, but not scrJE599A, repressed laf::lux activity relative to the elevated activity in an scrJ mutant or the wild type harboring the vector (Fig. 3E). We verified that the lack of a negative effect on laf::lux activity in strains expressing scrJE599A was not due to the absence of protein, as strains expressing ScrJ-GFP and ScrJE599A-GFP produced comparable levels of the fusion protein when analyzed by immunoblotting (Fig. S3A and B, lanes 3 and 4).
ScrL is another TPR-linked diguanylate cyclase that negatively affects swarming.
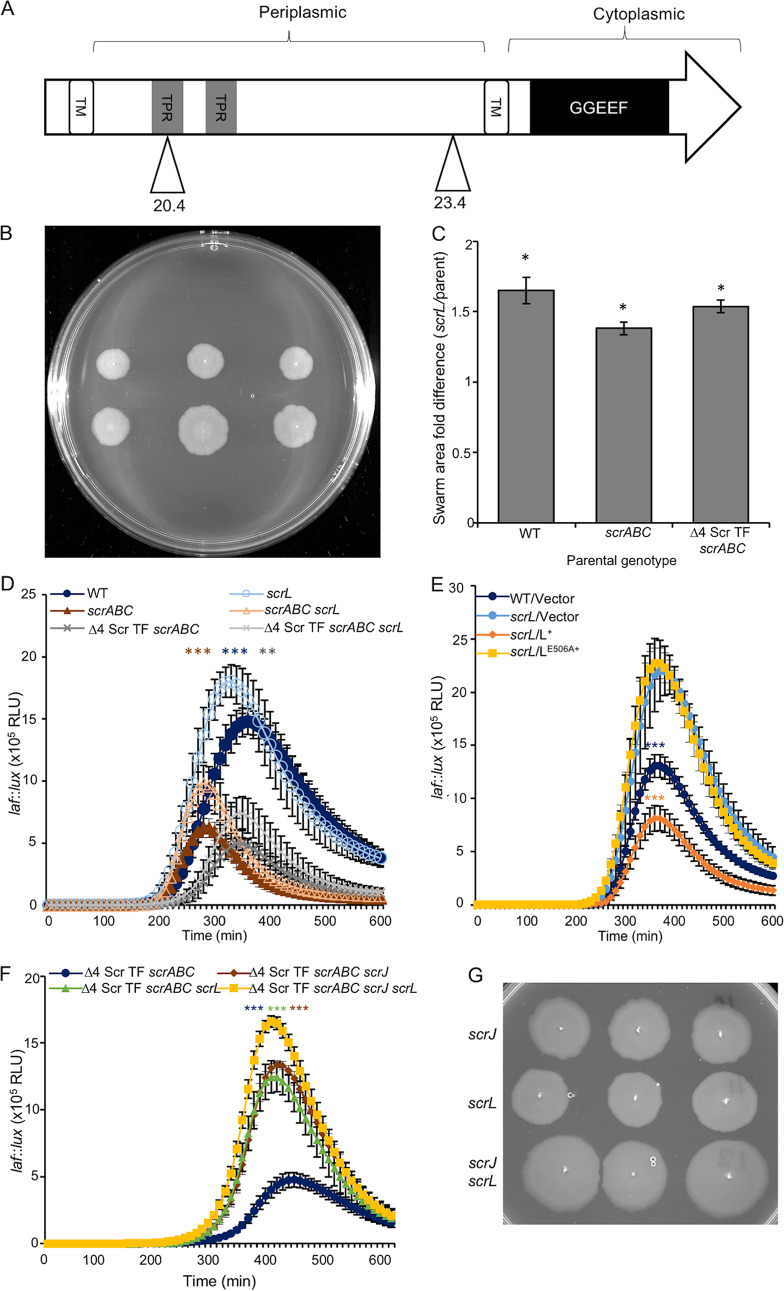
scrL (VPA1069) was identified twice in our screen with two unique insertions occurring in the LM11427 parent. Like ScrJ, ScrL (73.3 kDa) contains a predicted periplasmic region with three TPR domains and a cytoplasmic region with the putative GGDEF domain with the GGEEF motif (Fig. 4A). An scrL deletion was introduced into the Δ4 Scr TF scrABC strain LM11985. Increased swarming was observed, producing swarm areas ∼1.5 times larger than those of the parental strain and phenocopying the original transposon isolate phenotype (Fig. 4B and C). The effect of the scrL deletion on swarm migration and gene expression was also observed in wild-type and scrABC strains (Fig. 4C and D; Fig. S2C and D).
FIG 4.
ScrL is another diguanylate cyclase that negatively affects swarming. (A) Predicted domain organization of ScrL (73.3 kDa). Gray boxes indicate three putative tetratricopeptide repeat (TPR) domains. The white box denotes a putative transmembrane helix, and the black box represents the predicted GGDEF domain with the GGEEF catalytic motif. Triangles depict the sites of Tn5lux insertion obtained in our mutagenesis with their corresponding strain numbers. (B) Three swarming colonies for each strain, Δ4 Scr TF scrABC (LM11985) (top) and Δ4 Scr TF scrABC scrL (LM11997) (bottom). (C) Quantification of the ratios of swarm areas of the scrL mutants to the parent, scrL (LM11995)/WT (LM4476), scrABC scrL (LM11996)/scrABC (LM11984), and Δ4 Scr TF scrABC scrL (LM11991)/Δ4 Scr TF scrABC (LM11985). Bars indicate the average ratios of data from at least three experiments. Error bars denote standard errors, and pairwise comparisons were performed using Student’s t test (*, P < 0.05). (D to F) Lateral flagellar (laf::lux) gene expression during surface growth. (D) WT (LM1017 parental strain with flgE::lux), scrL (LM11992), scrABC (LM11524), scrABC scrL (LM11993), Δ4 Scr TF scrABC (LM11525), and Δ4 Scr TF scrABC scrL (LM11994) strains (n = 6). Pairwise comparisons of maximum RLU between scrL mutants and their isogenic scrL+ parents (e.g., LM1017 versus LM11992) were performed using Student’s t test (**, P < 0.01; ***, P < 0.001). (E) WT/V (LM12034), scrL/V (LM12038), scrL/scrL+ (LM12039), and scrL/LE506A (LM12040) strains. Strains were grown with 1 mM IPTG (n = 6). Pairwise comparisons between maximum RLU were performed against LM12038 using Student’s t test ( ***, P < 0.001). (F) Δ4 Scr TF scrABC (LM11525), Δ4 Scr TF scrABC scrJ (LM11988), Δ4 Scr TF scrABC scrL (LM11994), and Δ4 Scr TF scrABC scrJ scrL (LM12006) strains (n = 6). Pairwise comparisons of maximum RLU were performed against LM12006 using Student’s t test (***, P < 0.001). (G) Three single colonies from each strain, Δ4 Scr TF scrABC scrJ (LM11991), Δ4ScrTF ΔscrABC ΔscrL (LM11997), and Δ4 Scr TF scrABC scrJ scrL (LM12009). Fold increases in the swarm area ratios of double mutant strain LM12009 compared to LM11991 and LM11997 were 1.47 ± 0.07 and 1.50 ± 0.08, respectively (P < 0.01).
The E506A substitution mutation was introduced into the catalytic motif of ScrL to test ScrL’s diguanylate cyclase activity by changing the signature from GGEEF to GGAEF. Ectopically expressed scrL+ complemented the scrL mutant to decrease laf expression; however, scrL strains expressing the scrLE506A allele remained derepressed and exhibited laf gene activity similar to that of the scrL mutant harboring the vector control (Fig. 4E). The lack of a repressive effect by ScrLE506A was not the result of differences in protein production as levels of the wild-type and mutant forms were similar when immunoblotted for the GFP fusion protein (Fig. S3B, lanes 6 and 7). Therefore, like ScrJ, ScrL requires the canonical catalytic signature to regulate lateral flagellar gene expression.
When both mutations were combined in strains LM11525 and LM11985, the scrJ scrL double mutant achieved significantly higher peak laf::lux activity (Fig. 4F) and swarm areas (Fig. 4G), respectively, than either single mutant. The average double mutant swarm area was approximately 1.5 times the area of either single mutant. Thus, these two newly identified genes work independently to contribute to swarming regulation.
LafV, an EAL domain-containing protein, represses swarming.
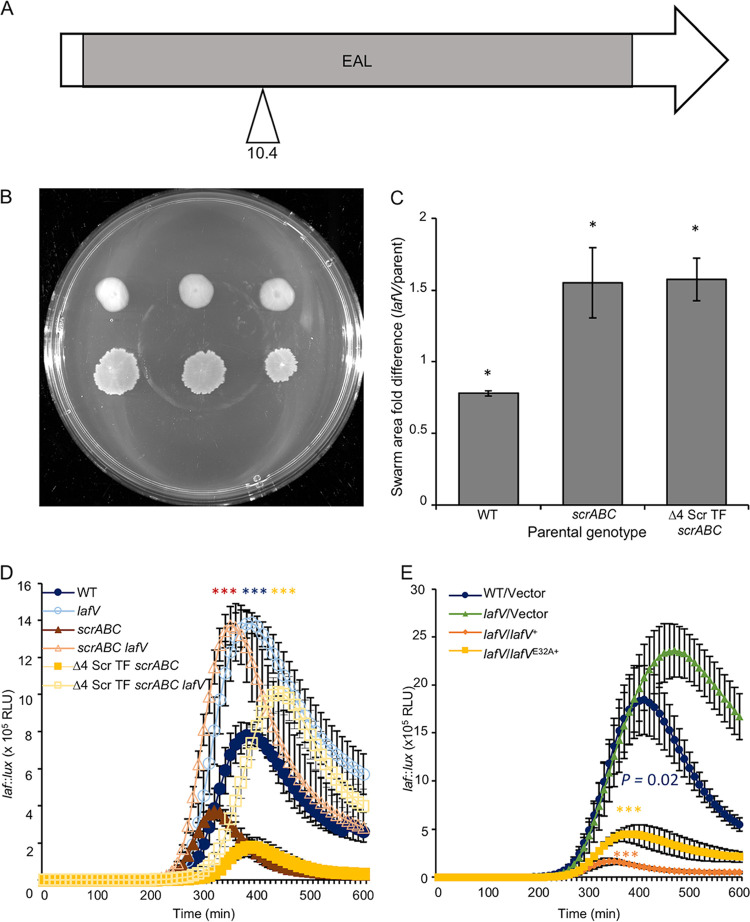
Contrary to the appearance of two DGCs in our mutagenesis screen, which is consistent with their predicted role in c-di-GMP synthesis, we were puzzled to discover a mutant with an insertion in a gene (lafV) coding for an EAL domain-containing protein. This lone mutant contained an insertion in the last gene (VPA1547) of the lateral flagellar C-ring- and export apparatus-encoding fliM operon. The ∼250-amino-acid EAL domain is the primary predicted feature of this ∼31-kDa protein (Fig. 5A). Introducing a lafV mutation into the Δ4 Scr TF scrABC and scrABC strains produced a modest swarm migration increase compared to the parental strains (Fig. 5B and C; Fig. S2F). Introducing this mutation into the wild type produced smaller swarms (Fig. 5C; Fig. S2E); however, there was significant derepression of laf::lux activity in all strain backgrounds (Fig. 5D). Furthermore, the profile of expression was perturbed over time: unlike the DGC mutants, which displayed elevated peak values, laf expression in lafV mutants remained elevated for the duration of the experiment compared to the wild type (compare Fig. 3D, Fig. 4D, and Fig. 5D).
FIG 5.
LafV is an EAL domain-containing protein that negatively affects swarming. (A) Predicted domain organization of LafV (31 kDa). The gray box indicates the putative EAL domain. (B) Three swarming colonies for each strain, Δ4 Scr TF scrABC (LM11985) (top) and Δ4 Scr TF scrABC lafV (LM12003) (bottom). (C) Quantification of the ratios of swarm areas of the indicated strain pairings (x axis) in swarm assays performed as described above for panel B in addition to the lafV (LM12001)/WT (LM4476) and scrABC lafV (LM12002)/scrABC (LM11984) strains. (D and E) Lateral flagellar (laf::lux) gene expression during surface growth. (D) WT (LM1017 with flgE::lux), lafV (LM11998), scrABC (LM11524), scrABC lafV (LM11999), Δ4 Scr TF scrABC (LM11525), and Δ4 Scr TF scrABC lafV (LM12000) strains (n = 6). Pairwise comparisons of maximum RLU between lafV mutants and their isogenic lafV+ parent (e.g., LM1017 versus LM11998) were performed using Student’s t test (**, P < 0.01; ***, P < 0.001). (E) WT/V (LM12049), lafV/V (LM12050), lafV/lafV+ (LM12051), and lafV/lafVE32A (LM12052) strains. Strains were grown with 100 μM IPTG (n = 6). Pairwise comparisons between maximum RLU were performed against LM12050 using Student’s t test (***, P < 0.001).
Although LafV (31 kDa) is annotated as an EAL domain-containing protein, the domain is poorly conserved (E value = 2.40e−05). The ubiquitous EAL domains, which are found in most bacteria, have been aligned into two subclasses that are hypothesized to be enzymatically active or inactive (4, 22, 23). In fact, there is precedent for a regulatory EAL-type protein controlling flagellar gene expression in Salmonella enterica serovar Typhimurium and Escherichia coli, i.e., RflP, also called YdiV (24–26). The paradoxical nature of LafV’s EAL domain and its negative effect on swarming suggested that LafV may function as a regulatory EAL protein and not a functional PDE. To probe this possibility, we introduced an E32A mutation into LafV, changing the EAL signature to AAL. Such an alteration has been introduced to inactivate other phosphodiesterases, including Vibrio cholerae VieA (27). For V. parahaemolyticus ScrC and ScrG, the EAL-to-AAL substitution eliminates laf gene activation and phenocopies the deletion of the entire EAL domain (10, 13). The ectopic production of wild-type LafV in a lafV mutant decreased laf::lux reporter activity, and the production of the LafVE32A version also repressed lateral flagellar gene expression, suggesting that catalytic activity was not integral to LafV’s regulatory activity (Fig. 5E).
LafV represses multiple genes in the surface-sensing regulon, including the major swarming regulator lafK.
Surface contact evokes the regulation of dozens of genes related to colonization, virulence, and persistence in the surface-sensing regulon (5). The genes comprising the surface-sensing regulon are organized into distinct regulatory units and expressed in three hierarchical classes (5, 15). Among these are the 38 laf genes encoding the lateral flagellar system. Since we had examined only the laf::lux fusion in the flagellar hook gene flgE, which resides in a class II operon, we were curious at what level in the hierarchy LafV exerted its negative regulatory effect. The lafV expression plasmid was introduced into strains containing lux fusions in fliH and flgM, which encode a component of the flagellar export apparatus and anti-σ28, respectively. Each of these genes is part of a unique transcriptional unit, and they represent class I (fliH) and class III (flgM) flagellar genes. The ectopic expression of lafV+ lowered the lux reporter activity of fliH and flgM compared to strains harboring the vector control (Fig. 6A). We also introduced the lafV+ expression plasmid into a reporter strain for VP1002, a nonflagellar class I gene in the surface-sensing regulon, encoding a large peptidase M60 domain-containing protein (5). Here, too, ectopic lafV+ expression significantly decreased the activity of the VP1002::lux reporter. The repressive activity of LafV was not general; the introduction of the expression clone into a control reporter strain with VP0829::lux, which encodes N-acetylglucosamine-6-phosphate deacetylase, failed to repress transcription of this control gene (Fig. 6A). Thus, LafV appears to exert its negative regulatory effect at the level of class I genes in the surface-sensing regulon.
FIG 6.
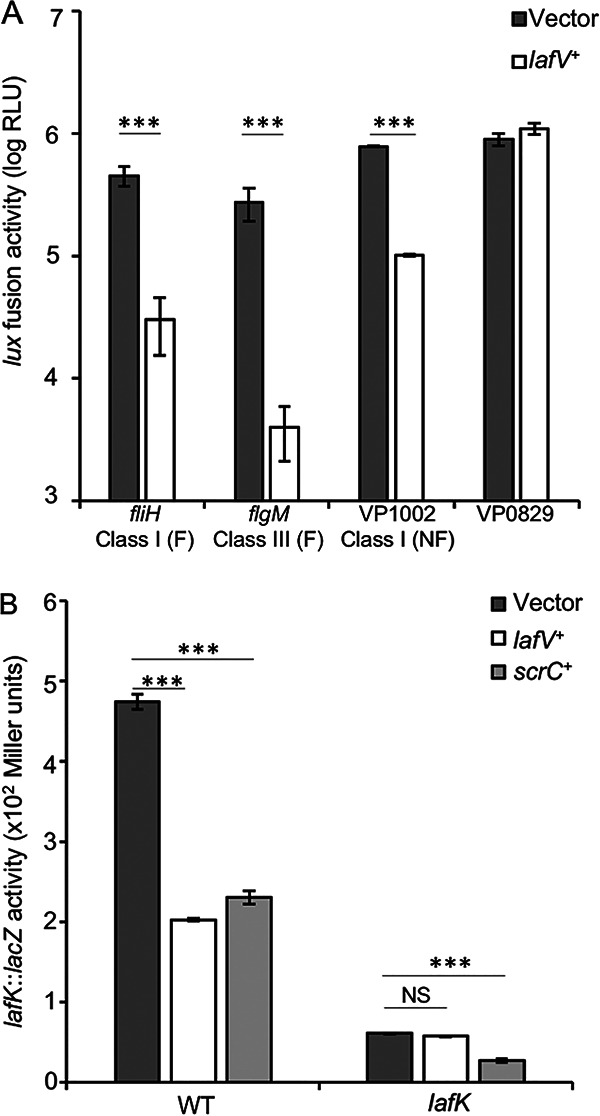
LafV negatively affects the expression of flagellar and nonflagellar genes in the surface-sensing regulon. Flagellar (F) and nonflagellar (NF) genes in the surface-sensing regulon are expressed in a hierarchy of three classes. (A) Strains bearing lux fusions in flagellar (fliH and flgM) and nonflagellar (VP1002) genes or a gene outside the surface-sensing regulon (VP0829) harboring either the vector (pLM1877) or a lafV+ expression plasmid (pLM3697). Reporter activity was measured at regular intervals during surface growth, and bars represent maximum reporter activities. Strains from left to right are LM12053, LM12054, LM12055, LM12056, LM12057, LM12058, LM12059, and LM12060. (B) The lafK::lacZ reporter strains LM10192 (WT) and LM7011 (lafK) harboring the vector (pLM1835) or an expression clone bearing lafV+ (pLM4710) or scrC+ (pLM3705) were grown on HI SW+ agar with 1 mM IPTG at 30°C and assayed for lacZ activity at 9 h. Strains from left to right are LM12044, LM12045, LM12046, LM12041, LM12042, and LM12043. For lux reporter fusions, values are the averages from 6 independent cultures, and lacZ reporters were measured in triplicate. In all panels, error bars represent standard deviations, and pairwise comparisons were performed between the indicated strains using Student’s t test (**, P < 0.01; ***, P < 0.001; NS, nonsignificant).
Much of the surface-sensing regulon is controlled by the σ54-dependent regulator LafK (5, 15, 28). Although LafK is not the “master” regulator of swarming, because it is not required for the expression of the fliM operon, it is responsible for the activation of many laf genes (5). LafK is encoded within a class I operon, it enhances its own operon’s expression, and it is required for class II gene expression (15). The repressive effect of LafV on lafK was examined by using a lafK::lacZ reporter plasmid. Reporter activity was depressed more than 2-fold upon lafV+ overexpression; however, repression was not observed in a strain lacking LafK (Fig. 6B). Although lafK::lacZ activity was low in the lafK mutant (in keeping with LafK’s autoregulatory role) and ectopic lafV+ expression failed to repress the lafK::lacZ reporter, this fusion could be regulated by the ectopic production of the diguanylate cyclase ScrC, which repressed lafK::lacZ in lafK+ and lafK backgrounds (Fig. 6B). Thus, LafV repression of lateral flagellar gene expression required LafK; moreover, c-di-GMP repression was found to be LafK independent.
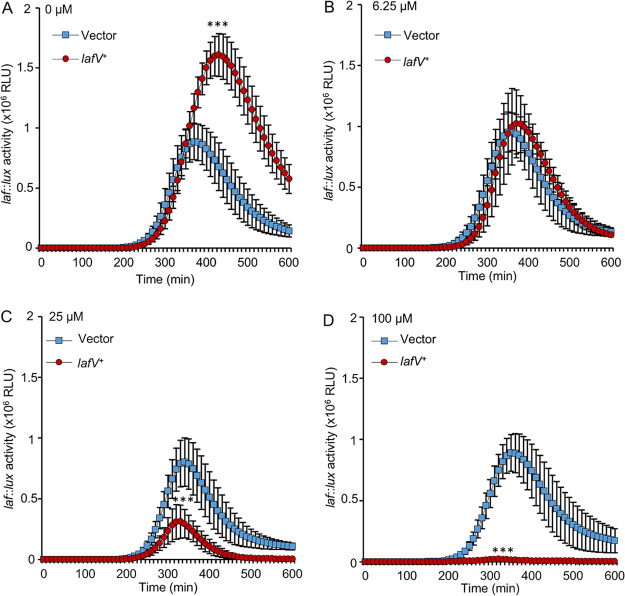
When ectopically produced, LafV repressed multiple genes in the surface-sensing regulon; when mutated, lafV mutants exhibited increased laf gene expression and suppressed the swarm-defective phenotype of the high-c-di-GMP scrABC strain. Not only was peak laf gene expression elevated in lafV mutants, but also the profile of swarming gene expression was altered, and transcription remained elevated during late-stage growth, unlike the effects of the DGC mutants (compare Fig. 3D, Fig. 4D, and Fig. 5D). To further probe the role of LafV, a titration experiment was performed using the IPTG-inducible lafV+ plasmid. In the absence of IPTG induction, strains harboring the lafV+ expression plasmid displayed an ∼50% increase in peak laf::lux activity compared to strains harboring the vector (Fig. 7A). While mild induction (6.25 μM IPTG) produced reporter activity similar to that when using the vector (Fig. 7B), further increases in the IPTG concentration resulted in more repressed laf reporter activity (Fig. 7C and D). These results suggest that small amounts of LafV may function to aid a strong swarming response; however, as LafV levels rise during the induction of the surface-sensing regulon, swarming gene expression ultimately gets turned down (producing the characteristic laf::lux profiles shown here). A negative-feedback role for LafV is consistent with the laf expression profiles of the mutant, which displayed elevated expression late in growth compared to its parental strain (Fig. 5D and E). Thus, LafV may play a dual positive and negative regulatory role by helping to coordinate swarming regulation. Although the loss of lafV leads to sufficiently increased laf gene expression to allow the suppression of c-di-GMP-mediated repression of swarming (Fig. 5B; Fig. S2F), this loss also leads to dysregulation: a failure to downregulate laf expression late in the swarming program (Fig. 5D) and impaired swarming motility in the wild type (Fig. 5C; Fig. S2E).
FIG 7.
LafV works positively and negatively to regulate lateral flagellar transcription. The laf::lux reporter strain LM1017 (flgE::lux) harboring the vector pLM1877 (LM12010) or the IPTG-inducible lafV+ expression clone pLM3697 (LM12011) was grown on a surface and monitored for light production using concentrations of IPTG of 0 μM (A), 6.25 μM (B), 25 μM (C), and 100 μM (D). Error bars represent standard deviations (n = 8). Pairwise comparisons of vector- and lafV+-expressing strains were performed at peak luminescence using Student’s t test (***, P < 0.001).
ScrJ and ScrL, but not LafV, affect the expression of the c-di-GMP-modulated reporter cpsA::lacZ.
Being predicted diguanylate cyclases that suppress the swarming defect of the high-c-di-GMP scrABC strain, which lacks a major swarm-relevant PDE in ScrC, ScrJ and ScrL appear to be key contributors to the c-di-GMP pool that controls swarming. This observation is further supported by data demonstrating that the site-directed mutations introduced into key residues in the GGDEF signature result in a loss of regulatory activity (Fig. 3E and Fig. 4E; Fig. S3A). If true, then scrJ and scrL mutant strains should also affect biofilm-pertinent gene expression in a reciprocal manner. In addition, the data suggest that LafV’s effect on swarming gene expression is regulatory and not catalytic; if true, then lafV mutation should not perturb c-di-GMP levels and should not influence biofilm gene expression. To test this, we employed the cpsA::lacZ fusion as a reporter of c-di-GMP-stimulated activity (Fig. 8). The transcription of cpsA is positively influenced by c-di-GMP (10, 11, 14). The introduction of scrJ or scrL was sufficient to reduce cpsA::lacZ gene expression, consistent with the predicted roles of these enzymes in synthesizing c-di-GMP. In contrast, but as expected in the context of a regulatory but not catalytically active protein, lafV mutation had no consequence for cpsA gene expression.
FIG 8.
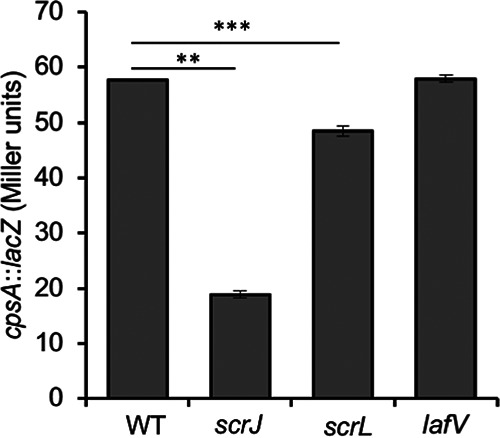
Capsule (cps) gene expression is diminished in scrJ and scrL mutants but is unaffected by the absence of lafV. Strains harboring the c-di-GMP-responsive cpsA::lacZ reporter on pLM3122 were grown for 12 h on HI SW+ agar and assayed for β-galactosidase activity. Strains from left to right are the WT (LM12061), scrJ (LM12062), scrL (LM12062), and lafV (LM12063) strains. Bars represent the averages from triplicate measurements, and error bars are standard deviations. Statistical significance was calculated using Student’s t test (**, P < 0.01; ***, P < 0.001).
DISCUSSION
The alternate lifestyles of swarming and sessility in V. parahaemolyticus are controlled by a complex network of environmental and social inputs that guide the activities of numerous regulators resulting in either swarming or biofilm formation. Underlying the choice of surface lifestyle is the concentration of the second messenger c-di-GMP. Two c-di-GMP-modulating enzymes in V. parahaemolyticus have been described to date, the hybrid DGC-PDEs ScrC and ScrG (7, 10, 13). Each of these proteins, although bearing both c-di-GMP-degradative and -synthetic capacities, functions primarily as a PDE under normal cellular conditions. Prior to the work described here, no DGCs had been identified as primary contributors to the c-di-GMP pool. Furthermore, while we previously established the importance of c-di-GMP levels in surface lifestyle selection and described key c-di-GMP binding transcription factors essential for properly executing the biofilm formation program, the link between high concentrations of c-di-GMP and swarming repression has remained elusive (11, 14). In this work, we add members to the current Scr network model (depicted in Fig. 1) by describing the activities of three new proteins contributing to swarming repression in V. parahaemolyticus. Two of these, ScrJ and ScrL, make straightforward contributions to swarming repression via c-di-GMP synthesis, while the third, LafV, is the first described degenerate EAL domain-containing regulatory protein in V. parahaemolyticus. Our work elucidates new members involved in the c-di-GMP network and provides new insight into flagellar gene regulation in V. parahaemolyticus.
ScrJ and ScrL are potent diguanylate cyclases in V. parahaemolyticus.
The mutagenesis screen identified two putative DGCs that appear to be major contributors to c-di-GMP synthesis in V. parahaemolyticus. ScrJ and ScrL rely on a GGEEF motif for their repressive function (Fig. 3E and Fig. 4E), and their deletion resulted in swarming derepression (Fig. 3D and Fig. 4D; see also Table S2 in the supplemental material). ScrJ and ScrL function additively (Fig. 4F and G). The predicted protein structures of these DGCs are intriguingly similar in their domain organizations, each residing in the inner membrane and possessing large periplasmic regions with multiple predicted TPR domains coupled to the cytoplasmic GGDEF domain. The presence of TPR domains in ScrJ and ScrL may indicate the potential to interact with other proteins, perhaps accessory regulators, localizing partners, or a complex of additional c-di-GMP-pertinent enzymes. To our knowledge, no such TPR-mediated interactions have been described for GGDEF or EAL domain-containing proteins, but there are numerous examples of the enzymatic activity of membrane-associated DGCs and PDEs being affected by protein-protein interactions. In Pseudomonas aeruginosa and Escherichia coli, the synthetic activities of the DGCs SadC and DgcE are stimulated by association with the flagellar stator protein MotC and the GTPase RdcA, respectively (29, 30). SadC’s DGC activity is enhanced by association with a dissociated MotC, which may function to push P. aeruginosa from a motile lifestyle further into its biofilm formation program (29). Conversely, DgcE, the major E. coli DGC responsible for initiating cellulose and curli production, is unable to self-dimerize in the absence of RdcA and is severely inhibited in extracellular matrix production and biofilm formation (30). Relatedly, the hybrid GGDEF-EAL PdeB, found in several Shewanella species (31), functions solely as a PDE but depends on the interaction of the inactive GGDEF domain with HubP for proper localization to the flagellated pole (32). While this interaction does not affect the activity of PdeB, it is possible that localization to the pole creates a low-c-di-GMP microenvironment within the area of the flagellum affecting motility through some posttranslational mechanism. Future work will focus on localizing and attempting to identify potential interaction partners for ScrJ and ScrL.
LafV may function to tune proper swarming initiation and repression.
We were surprised to discover a gene annotated as encoding an EAL domain-containing protein in our screen for swarming restoration, as such a mutation would be expected to increase intracellular c-di-GMP and thereby decrease swarming. That was not the case, as lafV mutations in our original parental recipient, which lacks scrABC and all four c-di-GMP binding Scr transcription factors, resulted in significant derepression of laf gene expression (Fig. 5D) and increased swarming (Fig. 5B and C; Table S2 and Fig. S2F). However, the domain is poorly conserved, and its repressive function was found to be independent of an intact catalytic EAL motif (Fig. 5E). Thus, LafV appears not to function as a PDE. This is further supported by examining cpsA::lacZ repression, which serves as a sensitive measure of cellular c-di-GMP: lafV mutation has no effect on cpsA gene expression (Fig. 8). This is in contrast to mutation of scrJ or scrL, which results in lower cpsA transcription levels and is consistent with a loss of diguanylate cyclase activity.
LafV’s role in swarming gene expression appears to be more complex than simple negative regulation, as the lafV mutant in the wild-type (i.e., scrABC+) background was less proficient in swarming motility than its parent strain (Fig. 5C; Fig. S2E), although laf gene expression was significantly elevated (Fig. 5D). We suspect that this complex phenotype is underscored by the importance of carefully coordinating the timing of flagellar expression and production. The low-level ectopic expression of lafV+ increased laf gene activation (Fig. 7A). However, further increasing the expression of lafV+ resulted in the repression of multiple genes in the surface-sensing regulon (Fig. 6A and Fig. 7C and D), including the swarming regulator lafK (Fig. 6B). Taken together, our results inform a model in which LafV may serve as an early enhancer of swarming, which, as LafV accumulates in the cell, shifts to a repressive role to provide feedback control for the swarmer cell. The existence of multiple and diverse regulatory mechanisms operating to carefully tune flagellar gene expression and morphogenesis is a common theme for almost all flagellar systems (33–36). For V. parahaemolyticus, which produces hundreds of lateral flagella, this regulation might be of particular importance.
Where does LafV exert its influence on swarming gene expression? No master swarming regulator has been identified, although LafK, which LafV represses, is essential for activating most swarming genes. Outside LafK’s regulon lies the fliM operon, which contains lafV, and other nonflagellar genes within the surface-sensing regulon. One of these lafK-independent nonflagellar genes, the putative peptidase-encoding VP1002 gene, is also repressed when lafV is overexpressed (Fig. 6A), suggesting that LafV may act near the top of the surface-sensing regulatory cascade.
How does LafV activate and repress swarming gene expression? LafV contains a single, predicted EAL domain. Since LafV failed to affect c-di-GMP-modulated biofilm gene expression and the glutamate residue in its EAL signature was not required for laf gene repression, our data point to LafV being a member of the subclass of EAL domain-containing proteins that function as catalytically inactive regulators (22). Similar to LafV’s role, RflP (formerly YdiV) negatively regulates motility in Escherichia coli and Salmonella enterica (24, 25). rflP expression is induced under conditions of nutrient depletion (37) and cell envelope stress (38) and functions to restrict flagellar gene expression by interacting directly with the FlhD4C2 master regulatory complex (24, 25) and targeting it for ClpXP-dependent proteolysis (26), thus repressing motility. Previous work implicated the Lon protease system (16) as a negative swarming regulator in V. parahaemolyticus, although the mechanism by which this repression occurs has remained elusive. LafK is not the master lateral flagellar regulator, nor does it share homology to FlhD4C2, and future work will focus on identifying potential interaction partners of LafV and the mechanism by which it exerts its dual regulatory effect.
Swarming in V. parahaemolyticus is a complex, energy-intensive phenotype requiring the activation of dozens of genes that results in the dramatic reorganization of cellular physiology and morphology. Improper initiation of this behavior could result in wasteful resource allocation and impose a fitness penalty on the cell. Similarly, there are times when biofilm formation may be strategic. Numerous sensory proteins feeding information about the environment into a tightly controlled regulatory network have evolved to ensure that the switch between motility and sessility is advantageous. Our work adds three new members of the surface colonization regulatory network and further reveals the intricacies involved in controlling this important lifestyle switch. Future work will focus on tracing the linkages in this network during growth on a surface by following up on the regulators described in this report as well as the other transposon mutants whose roles remain to be deciphered.
MATERIALS AND METHODS
Bacteria, growth media, and reagents.
Bacterial strains are described in Table S1 in the supplemental material. Strains were derived from V. parahaemolyticus BB22TR or BB22OP (9, 39). Plasmids were transformed into Escherichia coli strain DH5α (40) or π3813 (41) for genetic manipulations using pSW7848 (42). E. coli was grown in LB medium (43). V. parahaemolyticus was grown in heart infusion (HI) medium (Becton, Dickinson, Franklin Lakes, NJ) supplemented with 15 g liter−1 NaCl. Solid media were prepared with 20 g liter−1 granulated agar (Fisher Scientific) for nonswarming (SW−) conditions or with 15 g liter−1 granulated agar or 0.8% (wt/vol) agarose for surface-based (SW+) experiments on agar plates or microtiter plate assays, respectively. For transposon mutagenesis, conjugations were performed on HI medium without NaCl (HI no salt [NS]). For the selection of E. coli, chloramphenicol (Cam), kanamycin (Kan), gentamicin (Gen), and tetracycline (Tet) were added to LB at final concentrations of 20, 50, 15, and 10 μg ml−1, respectively. For the selection of V. parahaemolyticus, the concentrations of Cam, Kan, Gen, and Tet used were 2, 150, 25, and 5 μg ml−1, respectively, unless otherwise specified. For genetic manipulations using pSW7848-derived allelic exchange vectors, LB and HI media were supplemented with 1% d-glucose and 300 μM thymidine, and counterselection was performed using 0.2% l-arabinose. Experiments using derivatives of the inducible vectors pLM1835, pLM1877, and pDSW286 (44) were conducted using the specified concentrations of isopropyl-β-d-1-thiogalactopyranoside (IPTG; Research Products International, Mount Prospect, IL).
Molecular genetic techniques.
Plasmids and oligonucleotides are listed in Table S1, and the former were constructed using standard techniques and materials as described previously (45). Deletion/insertion mutations were made with a λ Red recombinase system in E. coli (46) to introduce mutations into the cosmid containing lafV to create pLM3700 using the antibiotic resistance cassettes found in plasmid pKD3. Silent deletions in scrJ, scrL, and lafV were constructed by amplifying upstream and downstream regions and inserted into pSW7848 via three-piece ligation to create the deletion constructs on plasmids pLM4703, pLM4704, and pLM4705, respectively. Mutations using plasmids pLM4703, pLM4704, and pLM4705 were constructed in V. parahaemolyticus using a method described previously by Val et al. (42). Catalytic E-to-A mutant variants scrJE599A and scrLE506A were created using overlap extension PCR and subsequently cloned, along with their wild-type counterparts, into EcoRI and BamHI sites of pLM3996 (pDSW286; from D. Weiss) to produce GFP-tagged versions of each protein.
Swarming restoration via Tn5lux mutagenesis.
The transposon Tn5lux (15) was employed to mutagenize two Vibrio strains, LM11427 and LM11430, using methods similar to those described previously by Enos-Berlage et al. (9). Equal volumes of cultures of donor strains carrying the suicide transposon plasmid pLM2819 and the tra donor pRK2013 and recipient Vibrio strains grown overnight were mixed, 10 10-μl aliquots per plate were spotted on multiple HI NS plates, and these conjugation plates were incubated overnight at 37°C. Each conjugation plate was considered an independent mutagenesis experiment; it was suspended in 5 ml HI broth and diluted ∼20-fold, and 100 μl was plated on multiple (2 to 4) HI SW+ plates with 5 μg ml−1 Cam and 75 μg ml−1 Kan to yield ∼100 colonies per plate. Plates were incubated at 30°C overnight and then allowed to develop for 1 to 2 days until swarming colonies were detected. Potential swarming-proficient mutants were purified and rescreened in comparison with the parental strain. Fifty independent mutagenesis experiments were performed, ∼15,000 colonies were screened, and 35 mutants were isolated. To prevent analysis of potential siblings, no more than 2 mutants from each mutagenesis experiment were saved for further characterization, except in cases where multiple mutants from a single mutagenesis experiment displayed distinct phenotypic characteristics.
The site of insertion for each transposon was mapped by sequencing arbitrarily primed PCR fragments (47) or Kan‐resistant clones that were derived by digestion and ligation of mutant chromosomal DNA and subsequent transformation of a λpir‐bearing E. coli strain.
Transcriptional reporter assays.
V. parahaemolyticus cultures grown overnight were diluted to an optical density at 600 nm (OD600) of 0.1 using a Genesys Spectronic 20 spectrophotometer (Thermo Fisher Scientific, Waltham, MA), and 100 μl was spread onto HI SW+ agar, incubated at 30°C, and measured at regular intervals. To assess reporter activity, 4 ml of HI broth was used to suspend plate growth, and strains were diluted to an OD600 of 0.1 to 0.4. For strains harboring lux reporter fusions, relative luminescence was measured in triplicate with a Promega (Madison, WI) Glomax Multi Jr. luminometer immediately following mixing to aerate the sample. Specific luminescence (SLU) was calculated as the luminescence per OD600 unit. For strains harboring lacZ fusions, β-galactosidase activity was measured in triplicate using a standard Miller assay as previously described (48).
For microtiter agar plate assays, 200 μl HI SW+ agarose was aliquoted into the wells of a 96-well black flat-bottom microtiter plate (Thermo Fisher Scientific, Waltham, MA). Strains were normalized to an OD600 of 0.05, and 4 μl was spotted into each well. The luminescence of wells was measured at 10-min intervals for 10 h in a Tecan Infinite M200 Pro plate reader (Tecan Group Ltd., Männedorf, Switzerland). To ensure similar growth among strains in all microplate-based experiments, the endpoint OD600 for wells was determined by removing the agarose plugs and vigorously vortexing the samples in 1 ml 1× phosphate-buffered saline (PBS). Resuspended cells were then measured against PBS vortexed with an uninoculated plug (data not shown). For experiments using the scrJ, scrL, lafVCam, and lafVGen expression plasmids, strains were grown overnight in HI Kan, HI Cam, or HI Gen broth and 100 μM IPTG before subculturing onto HI SW+ medium with IPTG at 1 mM (scrJ and scrL) or 100 μM (lafV).
Immunoblotting.
Cultures were grown overnight in 2 ml HI Kan broth with 1 mM IPTG. Cells were normalized to an OD600 of 0.1, and 100 μl was spread onto HI Kan SW+ agar with 1 mM IPTG and incubated for 8 h at 30°C, at which point cells were scraped and suspended in 4 ml of HI broth. Cultures were normalized and suspended in 2× Laemmli sample buffer (LSB) (49) to an OD600 of 3. Samples in LSB were heated at 95°C for 3 min. Ten microliters of each sample was loaded onto Novex WedgeWell 4 to 20% Tris-glycine 10-well gels (Invitrogen) and electrophoresed in running buffer (25 mM Tris, 0.2 M glycine, 3 mM SDS, 2.5% β-mercaptoethanol). Proteins were transferred onto Amersham Biosciences Hybond-P polyvinylidene difluoride (PVDF) membranes in transfer buffer (96 mM glycine, 12.5 mM Tris base, 20% methanol) using a Novex XCell II minicell (Invitrogen). Five percent nonfat dry milk in Tris-buffered saline–Tween 20 (TBS-T) (0.05% Tween 20, 0.15 M NaCl, 10 mM Tris-Cl [pH 8.0]) was used as the blocking solution. The primary antibody rabbit anti-GFP (catalog number 1083R-GFP3) was used at a 1:1,000 concentration and incubated with rocking overnight at 4°C. After thorough washing with TBS-T, the anti-rabbit secondary antibody conjugated with horseradish peroxidase (Amersham Biosciences) was added at a 1:20,000 concentration for 1 h at 4°C. After washing with TBS-T, the Clarity ECL substrate (Bio-Rad) was added according to the manufacturer’s instructions, and blots were imaged using the Bio-Rad ChemiDoc imaging system.
Graphical and statistical analyses.
Data were analyzed and plotted using Microsoft Excel. Images of swarming and measurements of swarm areas were prepared using Fiji (50). Statistical analyses were conducted using Student’s t tests. All data shown are representative of results from at least three independent experiments.
Supplementary Material
ACKNOWLEDGMENTS
We thank Casey Madrigal for performing the initial transposon mutagenesis and Megan Wassom, Brittney Dinkel, Evan Lamb, Floyd Evans, Jr., and Alex Dobrila for subsequent work characterizing mutant swarming and identifying sites of transposon insertion. We also thank David S. Weiss and Atsushi Yahashiri for providing pDSW286 and the anti-GFP antibody and Maria L. Morabe for assistance in performing the GFP tag immunoblotting. We thank all for helpful discussions.
J.H.K. was supported by NIH Mechanisms of Parasitism grant 5T32AI007511-24. Work in the McCarter laboratory is supported through a developmental grant from the University of Iowa’s Department of Microbiology and Immunology.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Jenal U, Reinders A, Lori C. 2017. Cyclic di-GMP: second messenger extraordinaire. Nat Rev Microbiol 15:271–284. doi: 10.1038/nrmicro.2016.190. [DOI] [PubMed] [Google Scholar]
- 2.Römling U, Galperin MY, Gomelsky M. 2013. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Römling U, Liang Z-X, Dow JM. 2017. Progress in understanding the molecular basis underlying functional diversification of cyclic dinucleotide turnover proteins. J Bacteriol 199:e00790-16. doi: 10.1128/JB.00790-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou S-H, Galperin MY. 2016. Diversity of cyclic di-GMP-binding proteins and mechanisms. J Bacteriol 198:32–46. doi: 10.1128/JB.00333-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gode-Potratz CJ, Kustusch RJ, Breheny PJ, Weiss DS, McCarter LL. 2011. Surface sensing in Vibrio parahaemolyticus triggers a programme of gene expression that promotes colonization and virulence. Mol Microbiol 79:240–263. doi: 10.1111/j.1365-2958.2010.07445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarter LL. 1999. The multiple identities of Vibrio parahaemolyticus. J Mol Microbiol Biotechnol 1:51–57. [PubMed] [Google Scholar]
- 7.Boles BR, McCarter LL. 2002. Vibrio parahaemolyticus scrABC, a novel operon affecting swarming and capsular polysaccharide regulation. J Bacteriol 184:5946–5954. doi: 10.1128/jb.184.21.5946-5954.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarter L, Silverman M. 1990. Surface-induced swarmer cell differentiation of Vibrio parahaemolyticus. Mol Microbiol 4:1057–1062. doi: 10.1111/j.1365-2958.1990.tb00678.x. [DOI] [PubMed] [Google Scholar]
- 9.Enos-Berlage JL, Guvener ZT, Keenan CE, McCarter LL. 2005. Genetic determinants of biofilm development of opaque and translucent Vibrio parahaemolyticus. Mol Microbiol 55:1160–1182. doi: 10.1111/j.1365-2958.2004.04453.x. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira RBR, Antunes LCM, Greenberg EP, McCarter LL. 2008. Vibrio parahaemolyticus ScrC modulates cyclic dimeric GMP regulation of gene expression relevant to growth on surfaces. J Bacteriol 190:851–860. doi: 10.1128/JB.01462-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimbrough JH, Cribbs JT, McCarter LL. 2020. Homologous c-di-GMP-binding Scr transcription factors orchestrate biofilm development in Vibrio parahaemolyticus. J Bacteriol 202:e00723-19. doi: 10.1128/JB.00723-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trimble MJ, McCarter LL. 2011. Bis-(3′-5′)-cyclic dimeric GMP-linked quorum sensing controls swarming in Vibrio parahaemolyticus. Proc Natl Acad Sci U S A 108:18079–18084. doi: 10.1073/pnas.1113790108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YK, McCarter LL. 2007. ScrG, a GGDEF-EAL protein, participates in regulating swarming and sticking in Vibrio parahaemolyticus. J Bacteriol 189:4094–4107. doi: 10.1128/JB.01510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira RBR, Chodur DM, Antunes LCM, Trimble MJ, McCarter LL. 2012. Output targets and transcriptional regulation by a cyclic dimeric GMP-responsive circuit in the Vibrio parahaemolyticus Scr network. J Bacteriol 194:914–924. doi: 10.1128/JB.05807-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart BJ, McCarter LL. 2003. Lateral flagellar gene system of Vibrio parahaemolyticus. J Bacteriol 185:4508–4518. doi: 10.1128/jb.185.15.4508-4518.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart BJ, Enos-Berlage JL, McCarter LL. 1997. The lonS gene regulates swarmer cell differentiation of Vibrio parahaemolyticus. J Bacteriol 179:107–114. doi: 10.1128/jb.179.1.107-114.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarter LL. 1998. OpaR, a homolog of Vibrio harveyi LuxR, controls opacity of Vibrio parahaemolyticus. J Bacteriol 180:3166–3173. doi: 10.1128/JB.180.12.3166-3173.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gode-Potratz CJ, McCarter LL. 2011. Quorum sensing and silencing in Vibrio parahaemolyticus. J Bacteriol 193:4224–4237. doi: 10.1128/JB.00432-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaques S, McCarter LL. 2006. Three new regulators of swarming in Vibrio parahaemolyticus. J Bacteriol 188:2625–2635. doi: 10.1128/JB.188.7.2625-2635.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malone JG, Williams R, Christen M, Jenal U, Spiers AJ, Rainey PB. 2007. The structure-function relationship of WspR, a Pseudomonas fluorescens response regulator with a GGDEF output domain. Microbiology (Reading) 153:980–994. doi: 10.1099/mic.0.2006/002824-0. [DOI] [PubMed] [Google Scholar]
- 21.Bobrov AG, Kirillina O, Ryjenkov DA, Waters CM, Price PA, Fetherston JD, Mack D, Goldman WE, Gomelsky M, Perry RD. 2011. Systematic analysis of cyclic di-GMP signalling enzymes and their role in biofilm formation and virulence in Yersinia pestis. Mol Microbiol 79:533–551. doi: 10.1111/j.1365-2958.2010.07470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Mouali Y, Kim H, Ahmad I, Brauner A, Liu Y, Skurnik M, Galperin MY, Römling U. 2017. Stand-alone EAL domain proteins form a distinct subclass of EAL proteins involved in regulation of cell motility and biofilm formation in enterobacteria. J Bacteriol 199:e00179-17. doi: 10.1128/JB.00179-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt AJ, Ryjenkov DA, Gomelsky M. 2005. The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains. J Bacteriol 187:4774–4781. doi: 10.1128/JB.187.14.4774-4781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wada T, Morizane T, Abo T, Tominaga A, Inoue-Tanaka K, Kutsukake K. 2011. EAL domain protein YdiV acts as an anti-FlhD4C2 factor responsible for nutritional control of the flagellar regulon in Salmonella enterica serovar Typhimurium. J Bacteriol 193:1600–1611. doi: 10.1128/JB.01494-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wada T, Hatamoto Y, Kutsukake K. 2012. Functional and expressional analyses of the anti-FlhD4C2 factor gene ydiV in Escherichia coli. Microbiology (Reading) 158:1533–1542. doi: 10.1099/mic.0.056036-0. [DOI] [PubMed] [Google Scholar]
- 26.Takaya A, Erhardt M, Karata K, Winterberg K, Yamamoto T, Hughes KT. 2012. YdiV: a dual function protein that targets FlhDC for ClpXP-dependent degradation by promoting release of DNA-bound FlhDC complex. Mol Microbiol 83:1268–1284. doi: 10.1111/j.1365-2958.2012.08007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tischler AD, Camilli A. 2004. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol Microbiol 53:857–869. doi: 10.1111/j.1365-2958.2004.04155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y-K, McCarter LL. 2004. Cross-regulation in Vibrio parahaemolyticus: compensatory activation of polar flagellar genes by the lateral flagellar regulator LafK. J Bacteriol 186:4014–4018. doi: 10.1128/JB.186.12.4014-4018.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker AE, Webster SS, Diepold A, Kuchma SL, Bordeleau E, Armitage JP, O’Toole GA. 2019. Flagellar stators stimulate c-di-GMP production by Pseudomonas aeruginosa. J Bacteriol 201:e00741-18. doi: 10.1128/JB.00741-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfiffer V, Sarenko O, Possling A, Hengge R. 2019. Genetic dissection of Escherichia coli’s master diguanylate cyclase DgcE: role of the N-terminal MASE1 domain and direct signal input from a GTPase partner system. PLoS Genet 15:e1008059. doi: 10.1371/journal.pgen.1008059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao L, Rakshe S, Leff M, Spormann AM. 2013. PdeB, a cyclic di-GMP-specific phosphodiesterase that regulates Shewanella oneidensis MR-1 motility and biofilm formation. J Bacteriol 195:3827–3833. doi: 10.1128/JB.00498-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossmann FM, Rick T, Mrusek D, Sprankel L, Dörrich AK, Leonhard T, Bubendorfer S, Kaever V, Bange G, Thormann KM. 2019. The GGDEF domain of the phosphodiesterase PdeB in Shewanella putrefaciens mediates recruitment by the polar landmark protein HubP. J Bacteriol 201:e00534-18. doi: 10.1128/JB.00534-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramanian S, Kearns DB. 2019. Functional regulators of bacterial flagella. Annu Rev Microbiol 73:225–246. doi: 10.1146/annurev-micro-020518-115725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guttenplan SB, Kearns DB. 2013. Regulation of flagellar motility during biofilm formation. FEMS Microbiol Rev 37:849–871. doi: 10.1111/1574-6976.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patrick JE, Kearns DB. 2012. Swarming motility and the control of master regulators of flagellar biosynthesis. Mol Microbiol 83:14–23. doi: 10.1111/j.1365-2958.2011.07917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simms AN, Mobley HL. 2008. Multiple genes repress motility in uropathogenic Escherichia coli constitutively expressing type 1 fimbriae. J Bacteriol 190:3747–3756. doi: 10.1128/JB.01870-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koirala S, Mears P, Sim M, Golding I, Chemla YR, Aldridge PD, Rao CV. 2014. A nutrient-tunable bistable switch controls motility in Salmonella enterica serovar Typhimurium. mBio 5:e01611-14. doi: 10.1128/mBio.01611-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spöring I, Felgner S, Preuße M, Eckweiler D, Rohde M, Häussler S, Weiss S, Erhardt M. 2018. Regulation of flagellum biosynthesis in response to cell envelope stress in Salmonella enterica serovar Typhimurium. mBio 9:e00736-17. doi: 10.1128/mBio.00736-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belas R, Simon M, Silverman M. 1986. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J Bacteriol 167:210–218. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 41.Le Roux F, Binesse J, Saulnier D, Mazel D. 2007. Construction of a Vibrio splendidus mutant lacking the metalloprotease gene vsm by use of a novel counterselectable suicide vector. Appl Environ Microbiol 73:777–784. doi: 10.1128/AEM.02147-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Val ME, Skovgaard O, Ducos-Galand M, Bland MJ, Mazel D. 2012. Genome engineering in Vibrio cholerae: a feasible approach to address biological issues. PLoS Genet 8:e1002472. doi: 10.1371/journal.pgen.1002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller JH. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 44.Schmidt KL, Peterson ND, Kustusch RJ, Wissel MC, Graham B, Phillips GJ, Weiss DS. 2004. A predicted ABC transporter, FtsEX, is needed for cell division in Escherichia coli. J Bacteriol 186:785–793. doi: 10.1128/jb.186.3.785-793.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silverman M, Showalter R, McCarter L. 1991. Genetic analysis in Vibrio. Methods Enzymol 204:515–536. doi: 10.1016/0076-6879(91)04026-K. [DOI] [PubMed] [Google Scholar]
- 46.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Toole GA, Kolter R. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol 28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 48.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 49.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 50.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.