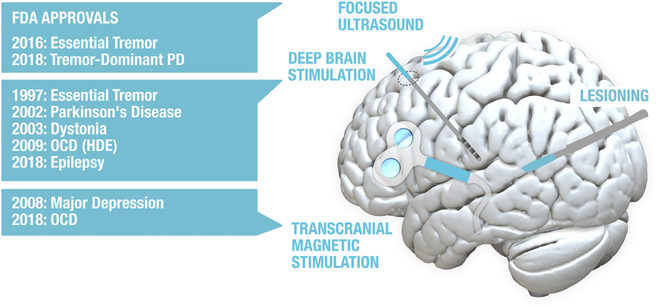
Fig. 1.
Methods used for clinical neuromodulation of the brain. List on the left shows recent device approvals issued by the U.S. Food and Drug Administration (FDA). HDE = Humanitarian device excemption. Various lesioning devices have been previously approved by the FDA for ablation of neural tissue (radiofrequency thermoablation, laser interstitial thermal therapy, sterotactic radiosurgery) with applications including thalamotomy for tremor, pallidotomy for Parkinson’s or dystonia, and cingulotomy for pain. Other technologies exist but have not been FDA approved for clinical neuromodulation of the brain (e.g. transcranial electrical current stimulation).