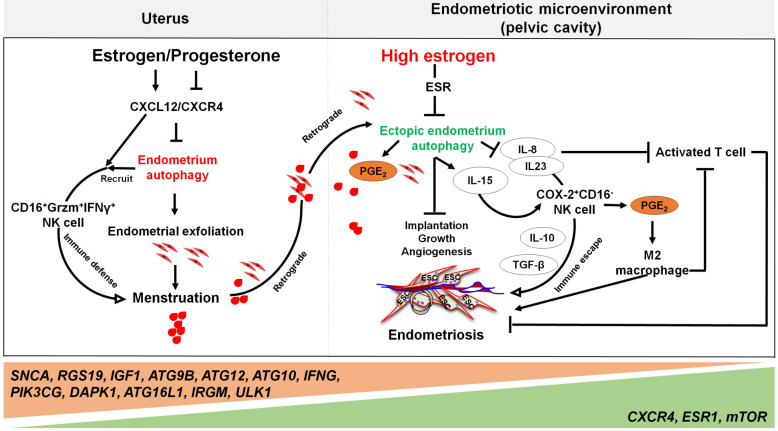
Figure 4.
The involvement of the ovarian steroid hormones-autophagy-immunity axis in the pathogenesis of endometriosis. In physiological situation, under the periodic regulation of estrogen and progesterone, autophagy levels in the endometrium also change in a CXCL12/CXCR4 dependent manner. During the menstrual period, the withdrawal of estrogen and progesterone leads to high levels of endometrial autophagy, contributing to endometrial exfoliation, immune defense, menstrual debris and endometrial fragment clearance by CD16+Granzyme B (Grzm)+INF-γ+ NK cells. In the presence of high concentrations of estrogen and progesterone resistance, however, autophagy-related genes (e.g., SNCA, RGS19, IGF1 ATGs, CXCR4, ESR1, and mTOR) are altered, leading to decreased endometrial autophagy. The suppression of endometrium autophagy directly accelerates the implantation, growth and angiogenesis of endometriotic lesions, whilst promoting the immune escape of endometriotic lesions through IL-8 and IL-23-mediated COX-2+CD16-NK cell differentiation. IL-15 is also involved in this process. Additionally, IL-8 inhibits activated T cells, and COX-2-induced PGE2 release stimulates M2 macrophage differentiation, which can facilitate the immune escape, remodeling of ECM and neovascularization of endometriotic lesions. Therefore, the effects of aberrant ovarian steroid hormones-autophagy-immunity axis contribute to the occurrence and development of endometriosis.