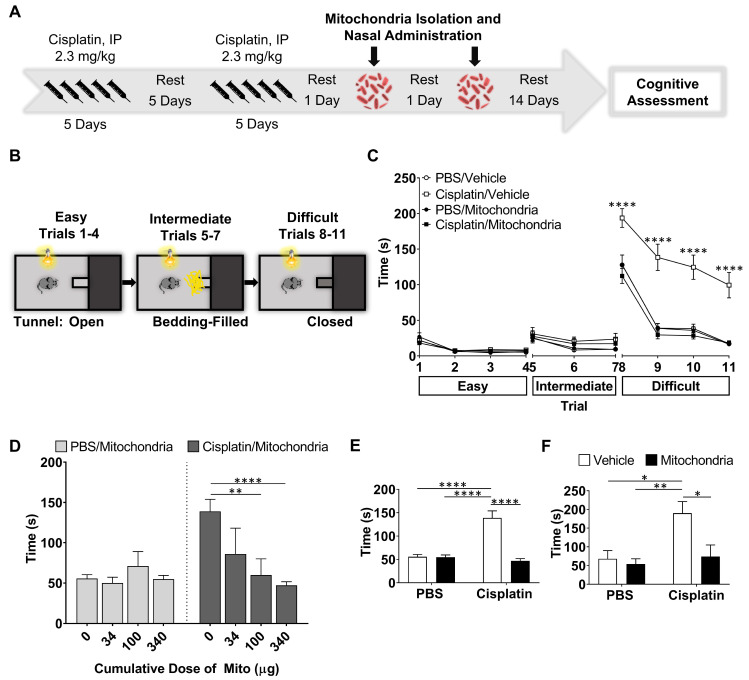
Figure 1.
Nasal administration of mitochondria derived from human MSC resolves cisplatin-impaired executive function. (A) Schematic representation of cisplatin-treatment regimen, mitochondria-based therapeutic strategy and cognitive testing. Mice were intraperitoneally injected with cisplatin at 2.3 mg/kg for 5 consecutive days, followed by 5 days of rest and another 5 days of cisplatin injection. On the second and fourth day following the last cisplatin injection, mitochondria were freshly isolated from human MSC and administered intranasally. 14 days later, their cognitive behavior was assessed. (B) Schematic representation of PBT for evaluating executive function. Mice in the bright chamber were subject to access the dark chamber at three levels of complexity - an open tunnel (easy trials 1-4), bedding-covered tunnel (intermediate trials 5-7) and plugged tunnel (difficult trials 8-11). (C) The time taken to enter the dark chamber was measured. Cisplatin-treated mice were either slow or failed to unplug the tunnel entry in the difficult trials. Mice, nasally administered with mitochondria performed efficiently (n = 16-22). (D) The restoration of the executive function was determined at 34, 100 and 340 µg of mitochondrial protein with significant effects at 340 µg, in the difficult trials (n = 4-22). Cisplatin-impaired executive function was resolved by nasal administration of mitochondria in both (E) male (n = 16-22) and (F) female mice (n = 6) as seen in the mean time taken to remove the plug and enter the dark chamber. Results are expressed as mean ± SEM; Two-way ANOVA with Tukey's post hoc analysis *p ≤ 0.05; **p ≤ 0.01; **** p ≤ 0.0001.