FIGURE 6.
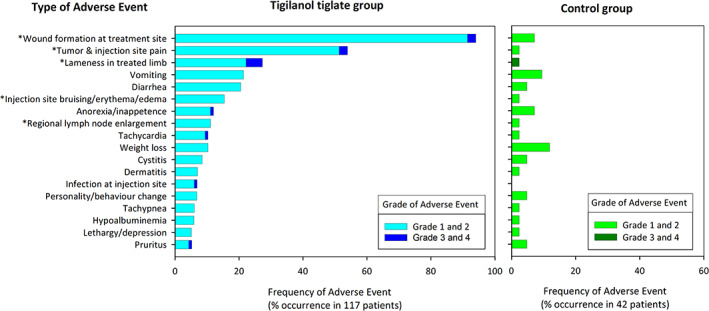
Occurrence and category of adverse events occurring at frequency of >5% in 117 dogs receiving a single treatment of tigilanol tiglate (phase 1 and 2 of the study) and the percentages of their occurrence in 42 control dogs (from phase 1 of the study). Asterisk (*) highlights adverse events that were ranked according to investigator opinion as definite or probably associated with the tigilanol tiglate treatment
