Abstract
miR-30c has been acknowledged as a tumor suppressor in various human cancers, such as ovarian cancer, gastric cancer, and prostate cancer. However, the role of miR-30c in glioblastoma (GBM) needs to be investigated. In our study, we found that the expression of miR-30c was significantly downregulated in GBM tissues and cell lines. We found that overexpression of miR-30c inhibited cellular proliferation of GBM cells in vitro and in vivo. More GBM cells were arrested in the G0 phase after miR-30c overexpression. Moreover, we showed that miR-30c overexpression suppressed the migration and invasion of GBM cells. Mechanistically, we found that SOX9 was a direct target of miR-30c in GBM cells. Overexpression of miR-30c inhibited the mRNA and protein levels of SOX9 in GBM cells. Moreover, there was a negative correlation between the expression of miR-30c and SOX9 in GBM tissues. Finally, we showed that restoration of SOX9 in GBM cells reversed the proliferation, migration, and invasion of GBM cells transfected with miR-30c mimic. Collectively, our results demonstrated that miR-30c suppressed the proliferation, migration, and invasion of GBM cells via targeting SOX9.
Key words: miR-30c, Proliferation, Migration, SOX9, Glioblastoma
INTRODUCTION
Glioblastoma (GBM) is the most common and malignant brain tumor to occur in the central nervous system of adults1,2. GBM is characterized with an increased malignant degree of invasion and growth3,4. Combined therapy of surgery, radiotherapy, and chemotherapy is generally used for GBM treatment. However, because of extreme aggression, the outcomes of GBM patients are quite poor5. The 5-year survival rate of GBM patients is very low6. Therefore, it will be of great significance to explore the underlying molecular mechanism of GBM development and progression.
MicroRNAs (miRNAs) are a class of small noncoding RNAs (ncRNAs). Previous studies have demonstrated that miRNAs could regulate gene expression through associating with the complementary site of the 3′-UTR of target mRNAs7,8. More and more studies show that miRNAs are widely involved in the regulation of a diversity of biological processes including cell proliferation, apoptosis, migration, and invasion9,10. Abnormal expression of miRNAs often leads to occurrence of cancers11–14. Liu et al. reported that miR-200c inhibits epithelial–mesenchymal transition, invasion, and migration of lung cancer by targeting HMGB112. Yang et al. reported that miR-483-5p promotes prostate cancer cell proliferation and invasion by targeting RBM513. Zeng and colleagues showed that miR-378 suppresses the proliferation, migration, and invasion of colon cancer cells by inhibiting SDAD114. miR-30c was also reported to inhibit tumor development. For example, Wu et al. showed that miR-30c negatively regulates the migration and invasion by targeting the immediate early response protein 2 in SMMC-7721 and HepG2 cells15. However, the function of miR-30c remains largely unknown in GBM. miRNAs have been demonstrated to be promising biomarkers and therapeutic targets for cancer intervention. Therefore, it is important to determine the mechanism of miRNAs in cancers.
In this study, we found that miR-30c was underexpressed in GBM tissues and cell lines. Moreover, overexpression of miR-30c significantly inhibited the proliferation, migration, and invasion of GBM cells. Mechanistically, we found that SOX9 is a direct target of miR-30c in GBM cells. We showed that overexpression of SOX9 could rescue the proliferation, migration, and invasion of GBM cells transfected with a miR-30c mimic. Taken together, our findings demonstrated the key role of miR-30c in GBM cells and explored the functional mechanism.
MATERIALS AND METHODS
Patient Samples
The present study was approved by the Ethics Committee of Linyi Central Hospital (Shandong Province, P.R. China), and written informed consent was obtained from patients with GBM for use of their tissues. A total of 53 paired GBM tissues and adjacent normal brain tissues were obtained from the patients with GBM who underwent surgical resection at Linyi Central Hospital. None of the patients were treated with radiotherapy or chemotherapy prior to surgery. Collected specimens were immediately frozen following surgical resection and stored in liquid nitrogen.
Cell Culture
Human GBM cell lines U87 and U251 obtained from the American Type Culture Collection (Manasas, VA, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; SH30022.01B; Hyclone, Logan, UT, USA) with 10% fetal bovine serum (FBS; Hyclone). Normal human astrocytes (NHAs) obtained from Lonza were cultured in the provided astrocyte growth media and 5% FBS.
Construction and Infection
miR-30c mimic and negative control (NC) were chemically synthesized by Ribobio (Guangzhou, P.R. China). The oligonucleotides of miRNA mimics and plasmids were transfected, according to the product specification, into the cells using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA).
Cell Proliferation
For the cell counting kit-8 (CCK-8) assay, stable transfected U87 and U251 cells were seeded in 96-well plates, and cells were cultured for 24, 48, 72, and 96 h before performing the CCK-8 assay (DOJINDO, Kumamoto, Japan). After incubation with CCK-8 at 37°C, absorbance (OD value) at a wavelength of 450 nm was detected and used for calculating cell viability.
For the colony formation assay, cells were harvested 24 h after transfection and then seeded in a new six-well plate (300 cells/well) and cultured for approximately 2 weeks until colony formation was observed. Colonies were fixed with methanol and stained with 1% crystal violet. A colony was considered to be 450 cells. Colony formation rate was used to calculate posttransfection cell survival rate.
In Vitro Invasion Assays
Cell invasion assay was performed using 24-well Transwell chambers with polycarbonate membranes containing 8-μm-diameter pores (Corning Incorporated, Corning, NY, USA). Cells were seeded on the top side of the membrane precoated with Matrigel in DMEM without serum (Becton Dickinson Company, Franklin Lakes, NJ, USA). The lower chambers were filled with DMEM and 10% FBS. After incubation at 37°C for 24 h, the noninvasive cells on the top side of the membrane were removed by scraping. Invasive cells on the lower membrane were fixed with 20% methanol for 30 min and stained with 0.1% crystal violet for 15 min. Invasion was quantified by counting cells in six randomized fields of view in each well under light microscope (Olympus, Tokyo, Japan) at the level of 100× magnification.
Reverse Transcription and Real-Time PCR
Total RNA of glioma samples, NHA samples, and cultured cells was extracted with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. The expression levels of SOX9 were assessed by SYBR green real-time quantitative reverse transcription PCR (RT-qPCR) and normalized with GAPDH.
Dual-Luciferase Reporter Assay
U87 cells were seeded into a 24-well plate. Cells were cotransfected with wild-type (WT), mutated SOX9 reporter plasmid or pMIR vector (Promega, Madison, WI, USA), and miR-30c mimic. Luciferase assays were conducted 24 h after transfection using the Dual-Luciferase Reporter Assay System (Promega).
Statistical Analysis
All data are shown as mean ± standard deviation (SD). Statistical significance was determined using Student’s t-test by SPSS 13.0 and GraphPad Prism 6. A value of p < 0.05 was considered statistically significant.
RESULTS
miR-30c Was Downregulated in GBM Cells
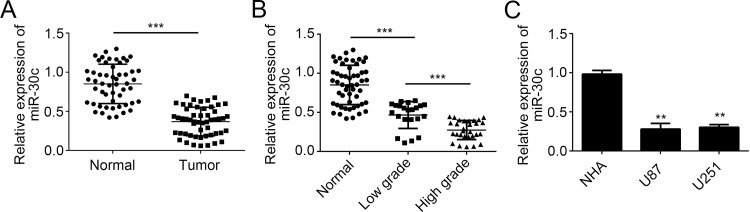
To investigate miRNA expression in human GBM tissues, we used RT-qPCR to evaluate the expression levels of miR-30c in 53 pairs of GBM tissues and adjacent normal tissues. We found that the expression of miR-30c was significantly downregulated in GBM tissues compared to normal tissues (Fig. 1A). Moreover, the decrease was more pronounced in high-grade GBM tissues compared to low-grade tissues (Fig. 1B). In addition, we chose GBM cell lines to analyze the expression of miR-30c by RT-qPCR. The results indicated that miR-30c was also downregulated in GBM cell lines compared with NHAs (Fig. 1C). These data indicated that miR-30c was downregulated in GBM tissues and negatively correlated with GBM malignance.
Figure 1.
MicroRNA-30c (miR-30c) was downregulated in glioblastoma (GBM) cells. (A) Relative expression of miR-30c in GBM tissues and paired normal tissues. (B) The expression of miR-30c in 53 noncancerous brain tissues, 22 low-grade glioma tissues, and 29 high-grade GBM tissues was measured by real-time quantitative reverse transcription PCR (RT-qPCR). (C) RT-qPCR analysis of miR-30c expression in GBM cell lines (U87 and U251 cells) and normal human astrocytes (NHAs). All data are representative of three independent experiments and expressed as mean ± standard deviation (SD). **p < 0.01 and ***p < 0.001.
Overexpression of miR-30c Suppressed the Proliferation of GBM Cells In Vitro and In Vivo
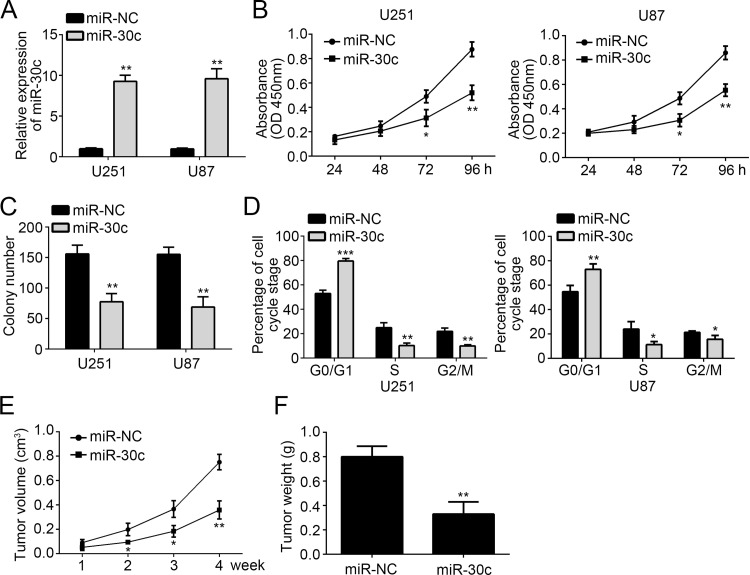
To explore the function of miR-30c in GBM, we overexpressed miR-30c in U251 and U87 cells by transfection with a miR-30c mimic. RT-qPCR analysis showed that the expression of miR-30c was significantly higher in U251 and U87 cells transfected with miR-30c mimic than miR-NC (Fig. 2A). Then we performed CCK-8 and colony formation assay to evaluate the effect of miR-30c on GBM cell proliferation. We found that overexpression of miR-30c significantly inhibited the cellular proliferation and colony numbers (Fig. 2B and C). Cell cycle distribution is directly linked to cell proliferation. We then explored the cell cycle distribution of U251 and U87 cells transfected with miR-30c mimic or miR-NC. Flow cytometry analysis (FACS) indicated that overexpression of miR-30c increased the percentage of cells in the G0/G1 phase and reduced the percentage in the S and G2/M phases (Fig. 2D). In order to determine whether miR-30c regulates tumor growth in vivo, we performed a xenograft experiment. At the indicated time points, we measured the tumor volumes and found that overexpression of miR-30c significantly inhibited tumor growth (Fig. 2E). Moreover, we checked the tumor weight at the end of the xenograft experiment and found that the tumor tissues derived from miR-30c-overexpressing cells were smaller (Fig. 2F). Therefore, our data demonstrated that overexpression of miR-30c suppressed cell proliferation in vitro and tumor growth in vivo.
Figure 2.
Overexpression of miR-30c suppressed the proliferation of GBM cells in vitro and in vivo. (A) The relative expression level of miR-30c in U251 and U87 cells was analyzed by RT-qPCR after transfection. (B) Overexpression of miR-30c inhibited cell proliferation detected by cell counting kit-8 (CCK-8) assay. (C) Long-term cell viability was evaluated using the colony formation assay. (D) The cell cycle phase of U87 and U251 cells transfected with miR-30c or negative control (miR-NC) was analyzed by flow cytometry. (E) Tumor volumes were measured at the indicated time points. (F) Tumor weight was determined at the end of experiments. All data are representative of three independent experiments and expressed as mean ± SD. *p < 0.05 and **p < 0.01 and ***p < 0.001.
Overexpression of miR-30c Inhibited the Migration and Invasion of GBM Cells
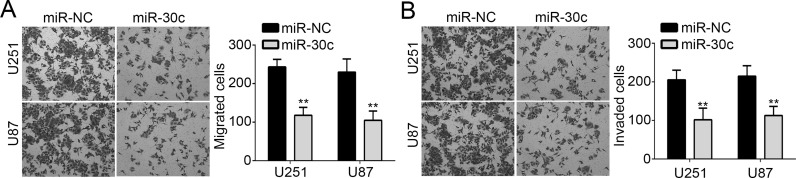
To assess the effect of miR-30c on cell migration and invasion, we performed the Transwell assay. The results demonstrated that overexpression of miR-30c significantly suppressed the migration and invasion of U251 and U87 cells (Fig. 3A and B). These results indicated that miR-30c may serve as a tumor suppressor in GBM progression.
Figure 3.
Overexpression of miR-30c inhibited the migration and invasion of GBM cells. (A, B) Cellular migration and invasion of U251 and U87 cells were determined by Transwell assay. All data are representative of three independent experiments and expressed as mean ± SD. **p < 0.01.
SOX9 Was a Target of miR-30c
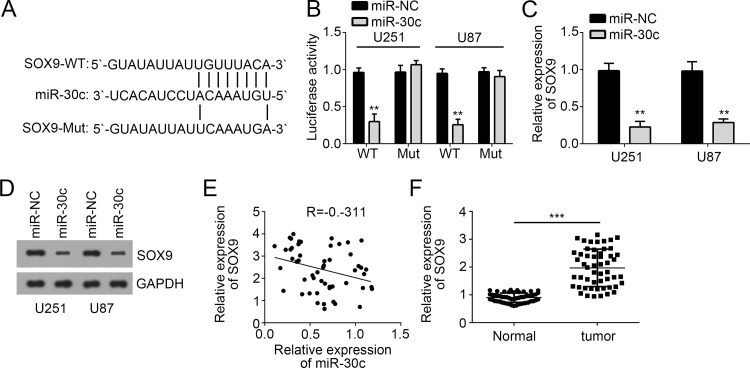
To explore the mechanism of miR-30c in GBM, we performed bioinformatics analysis to predict potential targets of miR-30c. Among all targets, SOX9 ranked top and serves as an oncogene in various tumors. There was a potential binding site of miR-30c in the 3′-UTR of SOX9 mRNA (Fig. 4A). Dual-luciferase reporter assays were conducted to verify their interaction. We found that overexpression of miR-30c significantly inhibited the luciferase activity in U251 and U87 cells transfected with SOX9-3′-UTR-WT, whereas mutation of the binding site abolished this effect (Fig. 4B). Moreover, through RT-qPCR and Western blot, we found that overexpression of miR-30c inhibited the mRNA (Fig. 4C) and protein levels (Fig. 4D) of SOX9 in U251 and U87 cells. Additionally, we also found that there was an inverse correlation between the expression of miR-30c and SOX9 in GBM tissues (Fig. 4E). Furthermore, through RT-qPCR analysis, we found that SOX9 expression was significantly upregulated in GBM tissues compared with adjacent normal tissues (Fig. 4F). Taken together, our results demonstrated that SOX9 was a direct target of miR-30c in GBM cells.
Figure 4.
SOX9 was a target of miR-30c. (A) Diagram for the miR-30c binding sites in the 3′-UTR of SOX9 with the wild-type (WT) and mutated (Mut) sequences. (B) U87 and U251 cells were cotransfected with miR-30c and luciferase reporter constructs containing either pGL3-SOX9-3′-UTR-WT or pGL3-SOX9-3′-UTR-Mut. (C) The mRNA and (D) protein levels of SOX9 in U87 and U251 cells transfected with miR-30c mimics were measured by RT-qPCR and Western blot. (E) Pearson’s correlation analysis of the relative expression levels of miR-30c and the relative mRNA levels of SOX9. (F) RT-qPCR analysis for SOX9 expression in GBM tissues and adjacent normal tissues. All data are representative of three independent experiments and expressed as mean ± SD. **p < 0.01 and ***p < 0.001.
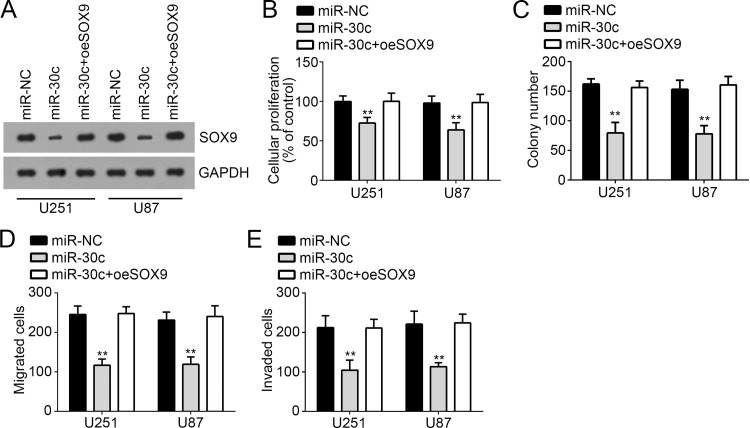
SOX9 Reintroduction Reverses the Inhibitory Effect of miR-30c
To further determine whether miR-30c inhibited the proliferation, migration, and invasion of GBM cells through targeting SOX9, we restored the protein level of SOX9 in U251 and U87 cells transfected with miR-30c mimic. By Western blot, we found that the expression of SOX9 was upregulated to the level of the control group (Fig. 5A). Then we performed CCK-8 and colony formation assays and found that overexpression of SOX9 really restored the proliferation ability of U251 and U87 cells and promoted colony formation (Fig. 5B and C). Moreover, the Transwell assay demonstrated that restoration of SOX9 in U251 and U87 cells transfected with miR-30c rescued the migration and invasion of GBM cells (Fig. 5D and E). Therefore, our findings indicated that miR-30c suppressed the proliferation, migration, and invasion of GBM cells through inhibiting SOX9 expression.
Figure 5.
SOX9 reintroduction reversed the inhibitory effect of miR-30c. (A) Protein levels of SOX9 in U251 and U87 cells were measured by Western blot. (B, C) Cell proliferation was measured by CCK-8 and colony formation assays. (D, E) Cell migration and invasion were determined by Transwell assay in U251 and U87 cells. All data are representative of three independent experiments and expressed as mean ± SD. **p < 0.01.
DISCUSSION
GBM is the most prevalent and aggressive brain cancer in adults, contributing to a large percentage of cancer-related deaths worldwide16. Nowadays, how GBM develops and progresses remains largely unknown. Therefore, there is a very urgent requirement to investigate the underlying mechanism of GBM progression. miRNAs have been widely acknowledged as pivot regulators in all kinds of biological processes and are closely linked with the development and progression of cancers17. miRNAs have been shown to be promising biomarkers for tumor diagnosis or prognosis18. Thus, understanding the molecular mechanism of miRNAs in the process of cancer development will be of great importance. In this study, we observed that miR-30c was downregulated in GBM tissues and cell lines and acted as a tumor suppressor.
miRNAs are thought to contribute to cancer occurrence due to their key function in the regulation of cell proliferation, apoptosis, and mobility. Dysregulation of miRNAs is often observed in various cancers. For example, miR-154 was downregulated in gastric cancer and inhibits the growth and metastasis of cancer cells by directly targeting MTDH19. Yang et al. reported that miR-494 is a potential prognostic marker and inhibits cellular proliferation, migration, and invasion by targeting SIRT1 in epithelial ovarian cancer20. In addition, Fan et al. showed that miR-122 was upregulated in clear-cell renal cell carcinoma and promoted metastasis of cancer cells by downregulating Dicer21. Previous studies indicated that miR-30c serves as a tumor suppressor in some tumors. For instance, Ni et al. showed that miR-30c suppressed giant-cell tumor of bone cell metastasis and growth via targeting HOXA122. Wang et al. reported that miR-30c inhibits metastasis of ovarian cancer by targeting metastasis-associated gene 123. Zhang and colleagues also indicated that low expression of miR-30c promotes prostate cancer cell invasion involved in the downregulation of KRAS protein24. Other reports also demonstrated the suppressive role of miR-30c in gastric cancer25, prostate cancer26, liver cancer15, and non-small cell lung cancer27. However, the role of miR-30c in GBM remains largely unknown. In our study, we demonstrated its low expression in GBM tissues and cell lines. Moreover, through CCK-8, colony formation, and Transwell assays, we demonstrated that overexpression of miR-30c significantly inhibited the proliferation, migration, and invasion of GBM cells, which suggested miR-30c also served as a tumor suppressor in GBM.
Increasing evidence indicates that SOX9 contributes to the progression of some cancers, including renal cell carcinoma28, non-small cell lung cancer29, hepatocellular carcinoma30, and GBM31. For example, a report indicated that SOX9-mediated upregulation of LGR5 is important for GBM tumorigenicity32. Another study showed that miR-145 functions as a tumor-suppressive RNA by targeting SOX9 and adducin 3 in human glioma cells33. However, the relationship between miR-30c and SOX9 remains elusive. In our study, we found that SOX9 was a direct target of miR-30c in GBM cells. Overexpression of miR-30c significantly inhibited the mRNA and protein levels of SOX9 in U251 and U87 cells. Moreover, we showed that there was a negative correlation between the expression of miR-30c and SOX9 in GBM tissues. Through functional experiments, we found that overexpression of SOX9 significantly reversed the effects of miR-30c on the proliferation, migration, and invasion of GBM cells, which suggested SOX9 was indispensable in miR-30c-mediated effects on GBM cells.
In summary, our findings demonstrated the key role of miR-30c on GBM cells. Our results indicated that miR-30c suppressed the development and progression of GBM through targeting SOX9, which provided a novel insight on the pathogenesis of GBM.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Miller CR, Perry A. Glioblastoma. Arch Pathol Lab Med. 2007;131(3):397–406. [DOI] [PubMed] [Google Scholar]
- 2. Easaw JC, Mason WP, Perry J, Laperriere N, Eisenstat DD, Del Maestro R, Belanger K, Fulton D, Macdonald D, Canadian Glioblastoma Recommendations Committee. Canadian recommendations for the treatment of recurrent or progressive glioblastoma multiforme. Curr Oncol. 2011;18(3):e126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith AW, Mehta MP, Wernicke AG. Neural stem cells, the subventricular zone and radiotherapy: Implications for treating glioblastoma. J Neurooncol. 2016;128(2):207–16. [DOI] [PubMed] [Google Scholar]
- 4. Binder DC, Davis AA, Wainwright DA. Immunotherapy for cancer in the central nervous system: Current and future directions. Oncoimmunology 2016;5(2):e1082027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khosla D. Concurrent therapy to enhance radiotherapeutic outcomes in glioblastoma. Ann Transl Med. 2016;4(3):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: The avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60(3):166–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flamini V, Jiang WG, Cui Y. Therapeutic role of miR-140-5p for the treatment of non-small cell lung cancer. Anticancer Res. 2017;37(8):4319–27. [DOI] [PubMed] [Google Scholar]
- 8. Wang JY, Jiang JB, Li Y, Wang YL, Dai Y. MicroRNA-299-3p suppresses proliferation and invasion by targeting VEGFA in human colon carcinoma. Biomed Pharmacother. 2017;93:1047–54. [DOI] [PubMed] [Google Scholar]
- 9. Si Y, Zhang H, Ning T, Bai M, Wang Y, Yang H, Wang X, Li J, Ying G, Ba Y. miR-26a/b Inhibit tumor growth and angiogenesis by targeting the HGF-VEGF axis in gastric carcinoma. Cell Physiol Biochem. 2017;42(4):1670–83. [DOI] [PubMed] [Google Scholar]
- 10. Zhou K, Luo X, Wang Y, Cao D, Sun G. MicroRNA-30a suppresses tumor progression by blocking Ras/Raf/MEK/ERK signaling pathway in hepatocellular carcinoma. Biomed Pharmacother. 2017;93:1025–32. [DOI] [PubMed] [Google Scholar]
- 11. Qu JJ, Qu XY, Zhou DZ. miR4262 inhibits colon cancer cell proliferation via targeting of GALNT4. Mol Med Rep. 2017;16(4):3731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu PL, Liu WL, Chang JM, Chen YH, Liu YP, Kuo HF, Hsieh CC, Ding YS, Chen WW, Chong IW. MicroRNA-200c inhibits epithelial-mesenchymal transition, invasion, and migration of lung cancer by targeting HMGB1. PLoS One 2017;12(7):e0180844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang ZG, Ma XD, He ZH, Guo YX. miR-483-5p promotes prostate cancer cell proliferation and invasion by targeting RBM5. Int Braz J Urol. 2017;43(6):1060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeng M, Zhu L, Li L, Kang C. miR-378 suppresses the proliferation, migration and invasion of colon cancer cells by inhibiting SDAD1. Cell Mol Biol Lett. 2017;22:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu W, Zhang X, Liao Y, Zhang W, Cheng H, Deng Z, Shen J, Yuan Q, Zhang Y, Shen W. miR-30c negatively regulates the migration and invasion by targeting the immediate early response protein 2 in SMMC-7721 and HepG2 cells. Am J Cancer Res. 2015;5(4):1435–46. [PMC free article] [PubMed] [Google Scholar]
- 16. Meyer MA. Malignant gliomas in adults. N Engl J Med. 2008;359(17):1850. [DOI] [PubMed] [Google Scholar]
- 17. Mei LL, Qiu YT, Wang WJ, Bai J, Shi ZZ. Overexpression of microRNA-1470 promotes proliferation and migration, and inhibits senescence of esophageal squamous carcinoma cells. Oncol Lett. 2017;14(6):7753–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao J, Li H, Liu L, Song L, Lv Y, Han Y. Identification and functional analysis of risk-related microRNAs for the prognosis of patients with bladder urothelial carcinoma. Oncol Lett. 2017;14(6):7297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qiao W, Cao N, Yang L. MicroRNA-154 inhibits the growth and metastasis of gastric cancer cells by directly targeting MTDH. Oncol Lett. 2017;14(3):3268–74. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20. Yang AJ, Wang XN, Yul CN, Jin ZZ, Wei LX, Ca JH, Wang Q, Zhang M, Zhang L, Zhang L, Hao CF. microRNA-494 is a potential prognostic marker and inhibits cellular proliferation, migration and invasion by targeting SIRT1 in epithelial ovarian cancer. Oncology Lett. 2017;14(3):3177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fan Y, Ma X, Li HZ, Gao Y, Huang QB, Zhang Y, Bao X, Du QS, Luo GX, Liu K, Meng QY, Zhao CF, Zhang X. miR-122 promotes metastasis of clear-cell renal cell carcinoma by downregulating Dicer. Int J Cancer 2018;142(3):547–60. [DOI] [PubMed] [Google Scholar]
- 22. Ni LY, Zhao JD, Lu YH, Li W, Li BL, Wang XC, Meng QG. MicroRNA-30c suppressed giant-cell tumor of bone cell metastasis and growth via targeting HOXA1. Eur Rev Med Pharmacol Sci. 2017;21(21):4819–27. [PubMed] [Google Scholar]
- 23. Wang X, Qiu LW, Peng C, Zhong SP, Ye L, Wang D. MicroRNA-30c inhibits metastasis of ovarian cancer by targeting metastasis-associated gene 1. J Cancer Res Ther. 2017;13(4):676–82. [DOI] [PubMed] [Google Scholar]
- 24. Zhang J, Wang XL, Wang YY, Peng RX, Lin ZY, Wang Y, Hu B, Wang JF, Shi GW. Low expression of microRNA-30c promotes prostate cancer cells invasion involved in downregulation of KRAS protein. Oncol Lett. 2017;14(1):363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cao JM, Li GZ, Han M, Xu HL, Huang KM. MiR-30c-5p suppresses migration, invasion and epithelial to mesenchymal transition of gastric cancer via targeting MTA1. Biomed Pharmacother. 2017;93:554–60. [DOI] [PubMed] [Google Scholar]
- 26. Huang YQ, Ling XH, Yuan RQ, Chen ZY, Yang SB, Huang HX, Zhong WD, Qiu SP. miR-30c suppresses prostate cancer survival by targeting the ASF/SF2 splicing factor oncoprotein. Mol Med Rep. 2017;16(3):2431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhong ZP, Xia Y, Wang PL, Liu B, Chen YJ. Low expression of microRNA-30c promotes invasion by inducing epithelial mesenchymal transition in non-small cell lung cancer. Mol Med Rep. 2014;10(5):2575–9. [DOI] [PubMed] [Google Scholar]
- 28. Hu B, Wang JB, Jin XB. MicroRNA-138 suppresses cell proliferation and invasion of renal cell carcinoma by directly targeting SOX9. Oncol Lett. 2017;14(6):7583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang S, Che D, Yang F, Chi C, Meng H, Shen J, Qi L, Liu F, Lv L, Li Y, Meng Q, Liu J, Shang L, Yu Y. Tumor-associated macrophages promote tumor metastasis via the TGF-beta/SOX9 axis in non-small cell lung cancer. Oncotarget 2017;8(59):99801–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Richtig G, Aigelsreiter A, Schwarzenbacher D, Ress AL, Adiprasito JB, Stiegelbauer V, Hoefler G, Schauer S, Kiesslich T, Kornprat P, Winder T, Eisner F, Gerger A, Stoeger H, Stauber R, Lackner C, Pichler M. SOX9 is a proliferation and stem cell factor in hepatocellular carcinoma and possess widespread prognostic significance in different cancer types. PLoS One 2017;12(11):e0187814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu N, Zhang L, Wang Z, Cheng YD, Zhang PX, Wang X, Wen WH, Yang HW, Liu H, Jin WL, Zhang YS, Tu YY. MicroRNA-101 inhibits proliferation, migration and invasion of human glioblastoma by targeting SOX9. Oncotarget 2017;8(12):19244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hiraoka K, Hayashi T, Kaneko R, Nasu-Nishimura Y, Koyama-Nasu R, Kawasaki Y, Akiyama T. SOX9-mediated upregulation of LGR5 is important for glioblastoma tumorigenicity. Biochem Biophys Res Commun. 2015;460(2):216–21. [DOI] [PubMed] [Google Scholar]
- 33. Rani SB, Rathod SS, Karthik S, Kaur N, Muzumdar D, Shiras AS. MiR-145 functions as a tumor-suppressive RNA by targeting Sox9 and adducin 3 in human glioma cells. Neuro-Oncology 2013;15(10):1302–16. [DOI] [PMC free article] [PubMed] [Google Scholar]