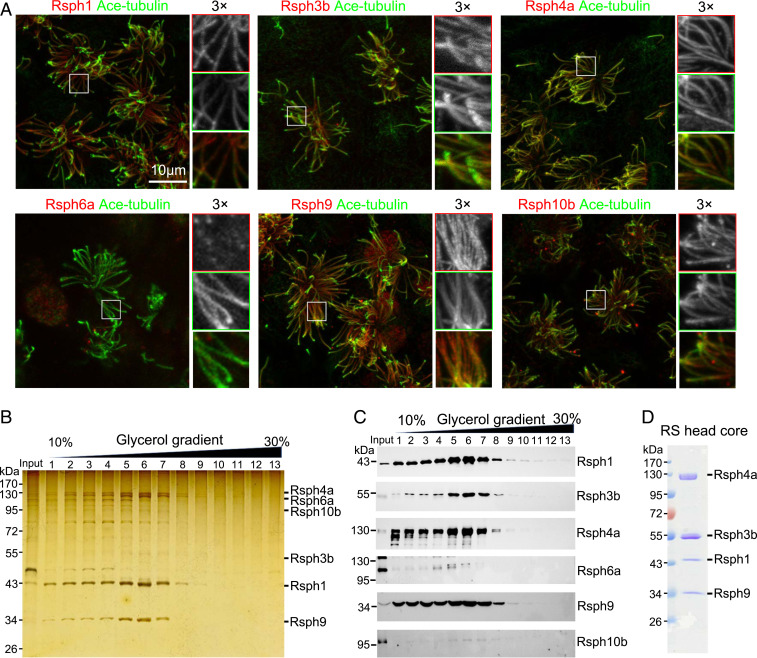
Fig. 1.
Rsph1–Rsph3b–Rsph4a–Rsph9 can form a stable RS head core complex and be purified. (A) Immunofluorescence of mEPCs showed that Rsph1, Rsph3b, Rsph4a, Rsph9, and Rsph10b, but not Rsph6a, localize in ciliary axonemes. Acetylated (Ace)-tubulin served as a ciliary axoneme marker. (B and C) Flag-tagged Rsph3b pulled down a complex abundant in Rsph1, -4a, and -9, but not -6a and -10b. The indicated proteins were coexpressed in HEK293T cells and coimmunoprecipitated with anti-Flag resins. The immunoprecipitates were subjected to a 10 to 30% glycerol gradient ultracentrifugation. Fractions collected from top to bottom were separated by SDS-PAGE, and followed by silver staining (B) and immunoblotting (C). Note that Rsph6a and Rsph10b were highly expressed in the input but poorly detected in the complex (C, fractions 5 to 7). (D) Purification of the Rsph1–Rsph3b–Rsph4a–Rsph9 complex by immunoprecipitation. The indicated proteins, in which Rsph3b and Rsph1, respectively, carried a Flag tag and a 6×His tag, were coexpressed in HEK293T cells and immunoprecipitated by using anti-Flag beads. The complex was detected by Coomassie blue staining after SDS-PAGE. This purification was repeated more than three times with similar results.