Graphical abstract
Keywords: Tetrapleura tetraptera, Astrocyte, Hypoxia, Oxidative stress, Binding affinity, Glutamine synthetase
Highlights
-
•
Aridanin, scopoletin, naringenin and ferulic acid were identified in methanol fruit extract of Tetrapleura tetraptera (TT) by HPLC-DAD.
-
•
TT demonstrated high antioxidant activity and radical scavenging ability in vitro.
-
•
TT maintained cellular redox status, viability and mitochondrial membrane potential.
-
•
Aridanin, from TT has a good binding affinity with glutamine synthetase in silico.
-
•
TT optimized glutamine synthetase so as to attenuate the severity of excitotoxicity.
Abstract
Oxidative stress and excitotoxicity are some of the pathophysiological abnormalities in hypoxia-induced brain injury. This study evaluated the intrinsic antioxidant property of methanol fruit extract of Tetrapleura tetraptera (TT), traditionally used for managing brain diseases such as cerebral infarction in West Africa, and its ability to protect primary astrocytes from anoxia-induced cell death. The effect of the phytochemicals present in TT on excitotoxicity was assessed in silico, through docking with human glutamate synthetase (hGS). Chromatographic and spectrophotometric analyses of TT were performed. Primary astrocytes derived from neural stem cells were treated with TT and its effect on astrocyte viability was assessed. TT-treated astrocytes were then subjected to anoxic insult and, cell viability and mitochondrial membrane potential were evaluated. Molecular docking of hGS with detected phytochemicals in TT (aridanin, naringenin, ferulic acid, and scopoletin) was performed and the number of interactions with the lead compounds, aridanin, analyzed. HPLC-DAD analysis of TT revealed the presence of various bioactive phytochemicals. TT demonstrated notable antioxidant and radical scavenging activities. TT also protected astrocytes from anoxic insult by restoring cell viability and preventing alteration to mitochondrial membrane integrity. Aridanin, naringenin, ferulic acid, and scopoletin demonstrated good binding affinities with hGS indicating that Tetrapleura tetraptera is a potential source of new plant-based bioactives relevant in the therapy of neurodegenerative diseases.
1. Introduction
Reactive oxygen species (ROS) are produced within the body due to normal metabolic activities and its harmful effects are counteracted by endogenous antioxidants. The imbalance between free radical production and antioxidant defense results in oxidative stress which causes deregulation of cellular functions and cause the development of various disease conditions. Oxidative stress has been implicated in various neurodegenerative diseases such as ischemic stroke and cerebral hypoxia [[1], [2], [3], [4], [5], [6], [7]].
Mitochondria, an important source of ROS in most cells [8] and their integrity is therefore of utmost importance. During oxidative phosphorylation, ATP synthase uses mitochondrial membrane potential (MMP) to produce ATP [9]. MMP provides the driving force for the production of cellular energy in the mitochondria, making ATP a compound buffering MMP. In hypoxic conditions, oxidative phosphorylation is halted, ATP generation is arrested and this leads to mitochondrial dysfunction [10].
Cerebral hypoxia is the reduction of cerebral oxygen and it is a part of physiological events involved in cerebral ischemic stroke [11,12]. Oxidative stress as a result of hypoxia causes mitochondrial dysfunction characterized by reduction in mitochondrial mass, change in membrane potential and change in oxidative proteins activities [5,8]. Cerebral hypoxia leads to various physiological dysfunctions such as oxidative stress and excitotoxicity, which culminate in the death of neuronal and non-neuronal cells [13]. In cerebral ischemia, excitotoxicity which is characterized by an increase in glutamate level accompanied by a decrease in glutamine level is observed. The decrement in the amount of glutamine has been explained to be due to the inhibition of glutamine synthetase [15]. Excitotoxicity induced marked expression of several pro-oxidant enzymes or mediators and therefore, alter brain synaptic plasticity and causes neurological dysfunction [16]. Glutamine synthetase catalyzes the condensation of glutamate and ammonia to form glutamine in an ATP-dependent mechanism [15] in other to mop up excess glutamate. Glutamine synthetase is highly expressed in astrocytes compared to other cells in the central nervous system [17].
Astrocytes are vital housekeeping non-neuronal cells of the central nervous system [18]. They perform a plethora of functions to maintain brain homeostasis. They scavenge the release of neurotransmitters during synaptic action, control the homeostasis of ions and water, release neurotrophic factors, transport metabolite and waste products, and participate in blood-brain barrier formation [18,19]. Breakdown of one or more of these astrocytes functions will either alone or in combination with other cell dysfunction, constitute a threat to neuronal survival [20]. Therefore, astrocytic cultures can be a useful model in determining whether the detrimental effects of anoxia can be overcome with therapeutic interventions.
In vitro models of neurological diseases have been reported to be useful for investigating the effects of preventive or therapeutic treatments on brain cells directly in both animal and human cultured cells [21,22].
Natural products such as medicinal plants contain pharmacologically active secondary metabolites [23]. These secondary metabolites are antioxidats and can safely interact with free radicals to stop the chain reaction that leads to damage of biomolecules through several mechanisms [[24], [25], [26], [27]].
Tetrapleura tetraptera fruit is effective for treating cerebral ischemia [28]. It is found in the lowland forest of tropical Africa such as Nigeria, Ghana, and Uganda, and has been traditionally used for managing brain diseases [29]. Its stem, bark, root, leaves, and fruits have various medicinal and nutritional properties [30]. The medicinal effects of Tetrapleura tetraptera plants have been ascribed to the presence of polyphenol components such as phenols, flavonoids and saponins [[31], [32], [33]]. The fruit of Tetrapleura tetraptera has been shown to have antihypertensive, anticonvulsive, and cognitive enhancing properties [[34], [35], [36], [37]].
We hypothesized that the methanol fruit extract of Tetrapleura tetraptera (TT) could be beneficial in scavenging free radicals and inhibiting lipid peroxidation that are implicated in cerebral ischemia and may probably be mechanisms used in protecting astrocytes from ischemia-induced mitochondrial biogenetic dysfunction.
Anoxic condition (chronic hypoxia) was employed in this study to mimic the oxygen deprivation in the situation of cerebral ischemia. In cerebral ischemia, the infarct zone has an ischemic core where the death of neurons is detected but the penumbra region (surrounding the ischemic core) does not immediately experience cell death and can be salvageable. In this study, we evaluate the antioxidative potential of TT to mitigate anoxia-induced bioenergetic disturbance in primary astrocytes, as well as examine the role of its phytoconstitutents to optimize human glutamate synthetase (hGS) in abating excitotoxicity through molecular docking.
2. Materials and methods
2.1. Chemicals
Bovine serum albumin (BSA), 2,3,5-triphenyl-1,3,4-triaza-2-azoniacyclopenta-1,4-diene chloride (TPTZ), thiobarbituric acid (TBA), ammonium molybdate, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), neocuproine, potassium ferricyanide, Trolox, sodium nitroprusside, 3-(4, 5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) and 5,59,6,69-tetrachloro-1,19,3,39-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other materials employed in this study were analytical grade and were purchased from standard suppliers within Nigeria.
2.2. Plant material and preparation of TT
Tetrapleura tetraptera fruits were obtained in the afternoon of April 2016 at the Department of Botany and authenticated at the same Department of Botany, Obafemi Awolowo University, Ile-Ife, Nigeria, in a geographical coordinates 07°30′59.46″ North and 04°31′42.82″ East (Voucher number: IFE17720). The fruits were sundried (65 % humidity), powdered and 600 g was soaked for 72 h in 70 % methanol with constant stiring at every 12 h. It was then sieved with a cloth of 100 μm pore size and further filtered with Whatman filter paper no. 1. The filtrate was concentrated in a rotary evaporator and further concentrated to dryness to obtain the extract (TT). The percentage yield of the extract was 11.6 % of dried plant material. It was dissolved in distilled water and DMSO for biological activity and cell culture experiments, respectively.
2.3. HPLC-DAD quantification of compounds in methanol fruit extract of Tetrapleura tetraptera (TT)
Compounds were quantified in TT using High Performance Liquid Chromatography-Diode Array Detector (HPLC-DAD) according to the previous method by Saliu et al. [28].
2.4. Evaluation of in vitro antioxidant activity
All experiments were run in triplicate and absorbance of the antioxidant assays were taken on a UV–vis spectrophotometer. Half maximal inhibitory concentration (IC50) of TT from the antioxidant assays was calculated using the inverse logarithmic method.
2.4.1. DPPH radical scavenging assay
The DPPH radical scavenging ability of TT was estimated using the method of Senguttuvan et al. [38]. This assay is based on the ability of polyphenol compounds to scavenge and neutralize DPPH radicals by donating one electron to the radical molecule [39]. DPPH radical solution (0.1 mM) was prepared in methanol and 0.5 mL was added to 0.5 mL of different concentrations of TT (10−100 μg/mL). The mixture was incubated in the dark at room temperature for 30 min. A control reference contained methanol instead of TT for baseline correction. After incubation, the absorbance (Abs) was taken at 517 nm. Ascorbic acid served as a standard antioxidant.
2.4.2. ABTS radical cation decolorization assay
The ability of TT to scavenge free radical was also determined by ABTS radical cation decolorization assay according to the method of Re et al. [40]. It is based on the ability of polyphenol compound to neutralize ABTS cation radicals by donating one electron to the radical molecule [39]. ABTS radical was produced by reacting 7 mM of ABTS and 2.45 mM potassium persulfate (1:1,v/v) and incubated in the dark at room temperature for 14 h. ABTS·+ solution was then diluted with 80 % methanol to obtain an absorbance of 0.700 at 734 nm. Then, 100 μl of different concentrations of TT or Trolox (12.5 μg/mL – 100 μg/mL) was added to 2 mL of diluted •ABTS·+ solution and the resulting mixture was incubated in the dark for 10 min and the absorbance (Abs) was taken at 734 nm. Control reference contained only ABTS•+ solution without TT for baseline correction. Percentage ABTS cation radical inhibition was calculated from the formula:
2.4.3. Hydroxyl radical scavenging activity
Hydroxyl (OH) radicals scavenging activity of TT was determined using the method described by Adjimani and Asare [41]. It is based on the ability of phytocompound to scavenge OH radicals by donating an electron to the radical molecule [39]. The Fenton reaction mixture was prepared by adding 0.2 mL FeSO4.7H2O (10 mM), 0.2 mL EDTA (10 mM), 0.2 mL 2-deoxyribose (10 mM) and 1.2 mL phosphate buffer (0.1 M, pH 7.4). Then, 0.2 mL of different concentrations of TT or mannitol (12.5 μg/mL – 100 μg/mL) were added, followed by the addition of 0.2 mL hydrogen peroxide (10 mM) and incubated for 4 h at 37ºC. To the incubated mixture, TCA (1 mL, 2.8 % w/v) and TBA (1 mL, 1 % w/v) were added and further incubated in boiling water for 10 min. Thereafter, it was allowed to cool at room temperature and then, centrifuged at 4000 rpm for 4 min. A control reference that contained all solution except TT for baseline correction was used. The absorbance (Abs) was measured at 532 nm against a blank solution.
2.4.4. Ferric reducing antioxidant power (FRAP) assay
Benzie and Strain [42] method was followed for determining FRAP value of TT. This assay is based on the diminution of ferric iron-TPTZ complex (Fe3+-TPTZ) by antioxidants to its ferrous form [39]. This reduction is monitored spectrohotometrically by measuring the change in absorbance at 593 nm. FRAP reagent solution containing 300 mM acetate buffer, 10 mM TPTZ in 40 mMHCl, 20 mM FeCl3.6H2O (10:1:1, respectively) was prepared. FRAP reagent (3 mL) was mixed with 100 μL TT (1 mg/mL) and incubated for 30 min incubation at 37ºC. The absorbance was then taken at 593 nm and FRAP value was extrapolated from a linear graph of FeSO4 and expressed as mmol Fe2+ equivalents per g dry weight.
2.4.5. Cupric ion reducing antioxidant capacity (CUPRAC) assay
CUPRAC assay was performed according to the method described by Apak et al. [43]. The ability of antioxidants to reduced Cu2+ to Cu+ in the presence of neocuproine [43]. The reduction is spectrohotometrically monitored at 593 nm. Copper(II) chloride (1 mL, 0.01 M), neocuproine (1 mL, 0.0075 M), and 1 M NH4CH3COO solution were added together. Then, 400 μL of TT (1 mg/mL) or freshly prepared Trolox solution of varying concentrations was added and diluted with 700 μL of deionized water. The mixture was incubated at room temperature for 30 min and the absorbance was taken at 450 nm. CUPRAC of TT was calculated with a molar extinction coefficient of 1.67 × 10−4 L mol−1cm−1 for Trolox using the formula:
Where absorbance of sample is the absorbance of TT.
2.4.6. Albumin anti-denaturation assay
Albumin anti-denaturation assay was carried out according to the method described by Sakat et al. [44]. The ability of polyphenols to hinder heat-induced protein denaturation was examined here. A reaction mixture containing 200 μL of 1 % bovine serum albumin (BSA) and 1 mL of varying concentrations of TT or ascorbic acid (as standard) was prepared, and the pH adjusted using 1 N HCl. The mixture was initially incubated at 37ºC for 20 min, then also at 60ºC for another 20 min, allowed to cool and the turbidity was measured at 660 nm. The control contained distilled water instead of TT or ascorbic acid. The percentage albumin denaturation inhibition was calculated as follows:
2.4.7. Lipid peroxidation assay
The assay for lipid peroxidation was carried out according to Ohkawa et al. [45]. This is a spectrophotometry assay that is based on the ability of polyphenol to inhibit peroxidation of polyunsaturated fatty acids of biological membrane through inhibiting the formation of thiobarbituric acid reactive substances, TBARS [46]. The excised brain of Wistar rat was homogenized in ice-cold 0.1 M phosphate-buffered saline (PBS, 10 %) and centrifuged at 3000 rpm for 10 min to obtain the brain supernatant. To 0.5 mL of the brain supernatant, 0.5 mL of TT or ascorbic acid of different concentrations (12.5−200 μl of 1 mg/mL) and 0.05 mL of freshly prepared FeSO4 (0.071 mM) were added, vortexed, and incubated at 37ºC for 30 min. After incubation, 1.5 mL of 0.8 % TBA in 1.1 % sodium dodecyl sulfate was added followed by another incubation at 95ºC for 1 h. It was allowed to cool and then centrifuged at 3000 rpm and the absorbance was taken at 532 nm. The control contained methanol instead of TT or ascorbic acid.
2.5. Evaluation of the effect of Tetrapleura tetraptera methanol fruit extract on astrocytes derived from Neural Stem Cells subjected to anoxia
2.5.1. Differentiation of primary human astrocytes from Neural Stem Cells
Astrocytes derived from human induced pluripotent stem cell (hiPSC) were used for this experiment. Human fetal brain tissue was procured following the informed consent of the mother. The brain tissues were processed according to approval by the Institutional Human Ethics and Stem Cell and Research Committee of National Brain Research Centre, India, in compliance with the Indian Council of Medical Research (ICMR). The telencephalon of human aborted fetuses (10–15 weeks old) was used to isolate fetal Neural Stem Cells (fNSCs). Isolation and differentiation of fNSCs to astrocytes were done as described by Fatima et al. [47] and Bhagat et al. [48].
Briefly, isolated neural stem cells (NSCs) were cultured in poly-d-lysine (Sigma-Aldrich, St. Louis, MO, USA) coated culture dishes in neurobasal media (Invitrogen, San Diego, CA, USA). The media was supplemented with Neural Survival Factor-1, N2 supplement, bovine serum albumin, glutamine, 25 ng/mL basic fibroblast growth factor (bFGF), 20 ng/mL epidermal growth factor (EGF), penicillin and streptomycin solutions, and gentamycin. The expression of neural stem cell markers, SRY-Box Transcription factor 2 (SOX2) and Nestin, and lineage-specific markers, Glial fibrillary acidic protein (GFAP) and microtubule-associated protein 2 (MAP2) were assessed for NSCs characterization using western blotting and immunocytochemistry. Almost 99 % of fNSCs showed immunoreactivity towards SOX2 and Nestin, and were negative for both GFAP and MAP2. Differentiation of NSCs into astrocytes was achieved by withdrawing neurobasal media and growing NSCs in miminum essential medium (MEM) supplemented with 10 % fetal bovine serum (FBS). Cells were maintained in MEM with FBS for atleast 21 days before using them for the experiments. Following trypsinization at the confluency of 80–90 %, cells were seeded for experiments in 8 well chamber slides or 12 well plates.
2.5.2. Astrocytes counting and seeding
The prepared astrocytes were counted to know the number of cells per volume of the media. Briefly, 10 μL of the cell was added onto a hemocytometer and viewed under the microscope for cell counting. The population of cells was then calculated:
For the 12 well plates, 100,000 cells were seeded; and for the 8 well chamber slide, 20,000 cells were seeded.
2.5.3. Induction of anoxia in astrocyte culture
Severe hypoxic condition (anoxia) was set by flushing the hypoxia chambers with a gas mixture of ∼0.2 % oxygen (replaced with 94.9 % nitrogen) and 5.1 % carbon dioxide [13], while normoxia chambers were maintained at 18.0 % oxygen and 5.1 % carbon dioxide and 76.9 % nitrogen, with a pressure of 1.75 bar for both conditions.
Viable but activated astrocytes were analyzed morphologically using the live/dead assay.
2.5.3.1. Assessment of astrocyte viability by live/dead assay
The live/dead assay is used for assessing cell viability/death and was carried out using a live/dead viability/cytotoxicity assay kit for mammalian cells (Invitrogen Detection Technologies, Thermofisher, UK) according to the manufacturer’s instructions. This method is used for assessing cell viability/death in live cell culture of cells. It uses two components, namely; Calcein AM (CaAM), which is component A and Ethidium homodimer-1 (EthD-1), which is component B. Dead cells are characterized by intense fluorescence at over 600 nm and little fluorescence around 530 nm.
Astrocytes seeded in an 8-well chamber slide were allowed to adhere and grow to confluence for 14 h at 37 °C in a CO2 incubator. Thereafter, cells were placed in a normoxic or severe hypoxic condition for 3 and 6 h. After 3 and 6 h, 100 μL of EthD-1 (1 μL/mL) and CaAM (0.2 μL/mL) were separately prepared in cell media (MEM), added to the cells, and then, incubated at 37ºC for 10 min. Fluorescent images of the cells were taken at excitation/emission(ex/em) wavelength of 495 nm/515 nm for the green filter (live cells) and ex/em wavelength of 495 nm/635 nm for the red filter (dead cells) using an Invitrogen Floid microscope (Fisher Scientific, Sweden). Percentage cell death was calculated to get the number of dead cells.
2.5.4. Determination of cytotoxicity of methanol fruit extract of Tetrapleura tetraptera on astrocyte cell culture
Astrocytes were seeded onto a 24 well-plate (Corning Flat Bottom Transparent Polystyrol well plate) at a density of 5 × 104 cells/well and were allowed to stay for 14 h at 37ºC in a CO2 incubator. The cells (n = 3) were treated for 24 h with 0.5, 1 and 10 μg/mL of TT dissolved in 0.01 % DMSO in the culture medium. Treatment with DMSO (0.01 % v/v culture medium) alone serves as the control. Thereafter, MTT assay was carried out.
MTT is a colorimetry method used for assessing cell viability and cytotoxicity for drug screening [49]. The MTT assay is based on the reduction of MTT (yellow colored) due to the activity of NAD(P)H-dependent oxidoreductase [50].
After 24 h of astrocyte treatment with the extract, 10 μl of MTT working solution (5 mg/mL) was added and then incubated in a CO2 incubator for 3 h at 37ºC. The medium was removed, and the formazan crystals formed were dissolved by adding 200 μl of DMSO and incubated in the dark inside a CO2 incubator for 1 h at 37ºC. Finally, the purple color of the dissolved formazan crystals was quantified in a plate reader (Infinite 200Pro Tecan, Switzerland) at 650 nm.
2.5.5. Evaluation of the viability of astrocyte treated with methanol fruit extract of Tetrapleura tetraptera after anoxia induction
Cells (astrocytes) were seeded in a 24 well-plate at a density of 5 * 104 cells/well (n = 3) and allowed to stay for 14 h at 37ºC in a CO2 incubator. Treatment with TT was performed 4 h before exposure to either 3 h normoxic (normoxia) or anoxic (anoxia) conditions. Two concentrations of TT (1 μg/mL and 10 μg/mL) were used and the treatments were as follows: cells treated with 1 μg/mL TT prior to anoxia (anoxia + TT 1 μg/mL); cells treated with 10 μg/mL TT prior to anoxia (anoxia + TT 10 μg/mL); cells treated with 10 μg/mL TT prior to normoxia (normoxia + TT 10 μg/mL). DMSO was used as a vehicle for TT.
After treatment, MTT assay was carried out to determine the percentage cell viability as previously described. Each assay was repeated twice.
2.5.6. Measurement of mitochondrial membrane potential of astrocytes after anoxia induction
The mitochondrial membrane potential (MMP) was measured by staining astrocytes with JC-1 (5,59,6,69-tetrachloro-1,19,3,39-tetraethylbenzimidazolyl-carbocyanine iodide) according to the method of Korenic et al. [51].
JC-1 is a ljipophilic and cationic dye that exhibits a fluorescence emission shift upon aggregation from 530 nm (green monomer) to 590 nm (red “J-aggregates’’ monomer). In healthy cells with high MMP, JC-1enters the mitochondrial matrix in a potential-dependent manner and forms aggregates.
Cells were seeded into groups as previously described. After treatment, cells were stained with 2.5 mg/mL JC-1 at 37ºC for 15 min. Thereafter, cells were rinsed three times with PBS and the dye was allowed to equilibrate at room temperature for another 10 min before imaging using an Invitrogen Floid microscope (Fisher Scientific, Sweden). Stained polarized mitochondria were detected with red fluorescence while the loss of mitochondrial integrity was detected with green fluorescence. The fluorescence was read on a plate reader (Infinite 200Pro Tecan, Switzerland) with an excitation wavelength of 485 nm and the absorbance at 530 nm and 590 nm. The JC-1 fluorescence values were first normalized separately to their respective controls for red and green signals before the relative MMP was calculated.
2.6. Molecular docking
Docking study was carried out in order to examine the activity of some phytcompounds in TT against excitotoxicity. Pathophysiological mechanisms observed in cerebral ischemic stroke are multifactorial and involve both neuronal and non-neuronal cell dysfunction [18,22,[52], [53], [54], [55]]. An important target enzyme in the pathway is glutamine synthetase [15,56,57]. Glutamine synthetase (GS) catalyzes the biosynthesis of glutamine from glutamate and ammonia. In the human brain, GS regulates excitotoxicity by converting neurotoxic glutamate (cause of excitotoxicity) to harmless glutamine with simultaneous hydrolysis of ATP [15].
2.6.1. Selection of protein and ligands
The 3D structure file of the identified target protein, human glutamine synthetase, hGS (Fig. 6), was retrieved from RCSB Server in the PDB format (PDB ID: 2ojw; www.rcsb.org).
Fig. 6.
Ribbon structure of Chain B from the homologous pentamer (A-E) of human glutamine synthetase (hGS) from RCSB (2ojw). The coloured part represents the funnel-shaped binding pocket.
Out of the compounds that were quantified from TT by HPLC, the most abundant phytochemical compounds from four different classes of phytoconstituents, namely, aridanin (saponin), ferulic acid (phenolic acid), naringenin (flavonoid), and scopoletin (coumarin) were selected as ligands (except aridanin which was selected based on the premise that it is a marker compound of Tetrapleura tetraptera). Their structures were adapted from PubChem NCBI database (https://www.ncbi.nlm.nih.gov/pubmed/), with ID number 73146, 1548883, 932, and 5280460, respectively (Fig. 7). The structures were delineated by ACD/ChemSketch 11.0 software and the MOL format of these ligands were generated. The structure of the ligands were brought to a ground state and PDB format generated by Avogadro 1.2 software.
Fig. 7.
3D structure of phytochemical constituent. (a) Scopoletin (pubchem ID: 5280460) (b) Aridanin (pubchem ID: 73146) (c) Ferulic acid (pubchem ID: 1548883) (d) Naringenin (pubchem ID: 932). Red balls: Oxygen atoms; White balls: Hydrogen atoms; Grey balls: carbon atoms; Blue ball: Nitrogen atom.
2.6.2. Optimization and docking of protein and ligand
Human glutamine synthetase was optimized for protein-ligand interaction studies by deleting all hetero atoms, ligands, and water molecules using Chimera software. The protein and ligands were loaded onto PyRx software in preparation for docking. The amino acid residue in the binding pocket, which includes the active site of hGS [58] was selected along with the ligands and toggled to set the grid parameters. The three-dimensional affinity grid parameters were set at 19.120, -13.908, -34.861 (x, y, z respectively) on the geometric center of the target protein and 40.177, 35.676, 36.044 (x, y, z respectively) as dimensions (Ǻ). Autodock vina from PyRx was used in docking the ligands into the binding pocket of the protein to get the binding energy data. Protein-ligand complex was generated using PyMol software in PDB format, the complex was uploaded on “Protein-Ligand Interaction Profiler (https://projects.biotec.tu-dresden.de/plip-web/plip)” and analyzed for the number of interactions formed [59].
2.7. Statistical analysis
All data were presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) followed by post hoc Tukey’s test was used for data analysis. Statistical significance was considered at values of p < 0.05. The statistical package used was GraphPad Prism 6 software (Graph Pad Software Inc., San Diego, CA, USA).
3. Results
3.1. Phytoconstituents
The analysis of compounds identified from the HPLC-DAD chromatogram of TT (Supplementary data) is presented in Table 1. It revealed the presence of isoliquiritigenin, naringenin, butein, scopoletin, aridanin, ferulic acid, echinocystic acid, umbelliferone, octodrine, piperazine and hentriacontane in TT. These compounds can be categorized into flavonoids, saponins, coumarins and phenolic acids. The most abundant phytochemical present was scopoletin (11.13 mg/g) and the least abundant were octodrine and isoliquiritigenin (0.17 and 0.12 mg/g, respectively).
Table 1.
HPLC-DAD quantified phytochemicals.
| Standard compound | Standard retention time | Methanol fruit extract of Tetrapleura tetraptera (TT) |
|
|---|---|---|---|
| Area (mUA) | (mg/g) | ||
| Ferulic acid | 1.27 | 1099.6020 | 1.38 |
| Echinocystic acid | 2.52 | 852.1330 | 1.07 |
| Umbelliferone | 2.75 | 1.384.0905 | 1.74 |
| Piperazine | 4.45 | 164.7690 | 0.21 |
| Aridanin | 4.70 | 285.5670 | 0.36 |
| Octodrine | 5.47 | 132.5145 | 0.17 |
| Scopoletin | 11.05 | 8866.8670 | 11.13 |
| Hentriacontane | 12.17 | 3123.0645 | 3.92 |
| Naringenin | 13.70 | 1581.5080 | 1.99 |
| Butein | 17.62 | 225.0670 | 0.28 |
| Isoliquiritigenin | 19.77 | 93.3595 | 0.12 |
3.2. Antioxidant activity of methanol fruit extract of Tetrapleura tetraptera
Antioxidant activity profile of TT shown in Fig. 1, Fig. 2 indicate the robust ability of TT to scavenge DPPH radicals (Fig. 1a), ABTS radicals (Fig. 1b), and hydroxyl radicals (Fig. 1c), as well as inhibit lipid peroxidation (Fig. 2a) and denaturation of albumin by heat (Fig. 2b) in vitro in a concentration-dependent manner. Table 2 showed the antioxidant activity, inhibition of lipid peroxidation, and inhibition of heat-induced denaturation of protein albumin of TT in the tests in terms of its half-maximal inhibitory concentration (IC50) in comparison with reference standards. TT demonstrated appreciable antioxidant activity with IC50 values of 20.96 ± 0.65, 47.03 ± 0.97, 20.63 ± 0.68 and 47.36 ± 0.67 for DPPH radical scavenging activity, ABTS radical scavenging activity, hydroxyl radical, and lipid peroxidation inhibitory activity, respectively, compared with the IC50 values of reference standards ascorbic acid (15.36 ± 0.29), Trolox (39.88 ± 0.52), mannitol (11.06 ± 0.87) and quercetin (45.61 ± 0.56), respectively. The IC50 of TT in the CUPRAC and anti-denaturation assays were 32.08 ± 1.22 and 26.76 ± 0.21, respectively, compared with the value of trolox (9.7553 ± 0.89) and ascorbic acid (12.15 ± 0.21), respectively, which were still appreciable. The FRAP value of the extract was 117.81 ± 4.066 mM Fe/mg dry weight while the TEAC value was 2.89 ± 0.451 mM Trolox/mg dry weight.
Fig. 1.
Scavenging activity of TT in vitro. (a) Percentage DPPH radical scavenging activity of TT; (b) Percentage ABTS•+ radical scavenging activity of TT; (c) Percentage hydroxyl radical scavenging ability of TT. Each bar represents the mean ± SD of three replicates. TT: Methanol fruit extract of Tetrapleura tetraptera; DPPH: 2,2-diphenyl-1-picrylhydrazyl; ABTS: 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid).
Fig. 2.
Inhibitory activity of TT against lipid peroxidation and protein denaturation. (a) Percentage lipid peroxidation inhibitory effect of TT in rat brain homogenate; (b) Percentage inhibitory effect of TT on denaturation of albumin. Each bar represents the mean ± SD of three replicates. TT: Methanol fruit extract of Tetrapleura tetraptera.
Table 2.
IC50 values of Tetrapleura tetraptera methanol fruit extract in in vitro antioxidant tests.
| Antioxidant tests | TT IC50 (μg/mL) | Reference standard |
|
|---|---|---|---|
| Compound | IC50 (μg/mL) | ||
| DPPH | 20.96 ± 0.65a | Ascorbic acid | 15.36 ± 0.29b |
| ABTS•+ | 47.03 ± 0.97a | Trolox | 39.88 ± 0.52b |
| Hydroxyl radical | 20.63 ± 0.68a | Mannitol | 11.06 ± 0.87b |
| CUPRAC | 32.08 ± 1.22a | Trolox | 9.76 ± 0.89b |
| LPO | 47.36 ± 0.67a | Quercetin | 45.61 ± 0.56b |
| Protein anti-denaturation | 26.76 ± 0.21a | Ascorbic acid | 12.15 ± 0.21b |
Values are expressed as mean ± SD (n = 3). IC50 was calculated using the inverse logarithmic method. Values with the same letter across a row are not significantly different (p < 0.05). TT: Tetrapleura tetraptera methanol fruit extract; DPPH: 2,2-diphenyl-1-picrylhydrazyl; ABTS: 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid); CUPRAC: Cupric ion reducing antioxidant capacity; LPO: Lipid peroxidation.
3.3. Cell death after anoxia
The survival rate of astrocytes following anoxic conditions (severe hypoxia) is shown in Fig. 3. When astrocytes were subjected to anoxia for 3 h (Fig. 3a), the cell death was 15 % compared to normoxia (p < 0.01), and when the severe hypoxic exposure was for 6 h, 55 % of astrocytic cell death occurred (Fig. 3b) compared to that in the normoxia condition (p < 0.01). With 3 h anoxia exposure, a larger number of astrocytes with altered star shape were observed suggesting that they were morphologically impaired live cells. Therefore for further experiments, the 3 h anoxia where cells were morphologically impaired with less cell death was used because it was important to have morphologically impaired viable cells to test the neuroprotective property of TT.
Fig. 3.
Measure of astrocyte survival following hypoxic treatment. (a) Effect on survival of astrocytes after 3 h exposure to (i) normoxicor (ii) anoxic conditions. (b) Effect on survival of astrocyte after 6 h of (i) normoxic or (ii) anoxic conditions. Each bar is expressed as mean ± SD (n = 3). Significance with student t-test indicated **p < 0.01. All the cells that appeared red (bright or dull) are dead cells while most of the green cells are live cells. Some cells which appeared green but had round shape were counted as dead cell because the round shape indicated that cells were dying. Green cells with altered star-shape were the astrocytes that have lost their morphology and were counted as live cells.
All the cells that appeared red (bright or dull) are dead cells while most of the green cells are live cells. Some cells which appeared green but had round shape were counted as dead cell because the round shape indicated that cells were dying. Green cells with altered star shape were the astrocytes that have lost their morphology and were counted as live cells.
3.4. Cytotoxicity of methanol fruit extract of Tetrapleura tetraptera on astrocyte
Cytotoxicity of TT on astrocytes after 24 h of treatment is shown in Fig. 4a. Compared with the control (0.01 % DMSO), the three concentrations of TT (0.5 μg/mL, 1.0 μg/mL and 10.0 μg/mL) showed no cytotoxicity (p < 0.05). This implied that the extract did not have deleterious effects on the viability of astrocytes up to10 μg/mL.
Fig. 4.
Effect of methanol fruit extract of Tetrapleura tetraptera (TT) on cell viability using the MTT method. (a) Effect of TT on cell viability of astrocytes. Astrocytes were treated for 24 h with varying concentrations of TT to assess its cytotoxicity using MTT method. There was no cell death observed at all doses. Bars with the same letters are not significantly different (p < 0.05) compared to control. (b) Effect of TT pretreatment on the viability of astrocytes after 3 h normoxia and anoxia. A significant increase in viable cells indicated that astrocytes were protected during an anoxic condition when pretreated with TT. Each bar in (a) and (b) is expressed as mean ± SD (n = 3). #p < 0.05 vs normoxia; * p < 0.05 vs anoxia. TT: Methanol fruit extract of Tetrapleura tetraptera.
3.5. Effect of methanol fruit extract of Tetrapleura tetraptera on cell viability following 3 h anoxia
There was a significant reduction (p < 0.05) in viable astrocytes after 3 h of anoxia treatment compared to control (Fig. 4b). This showed that anoxia may result in a reduction of metabolic activity which could be a contributory mechanism in causing the death of cells. When cells were treated with TT before subjecting them to 3 h anoxic condition, the total number of viable cells was significantly higher (p < 0.05) than untreated cells. This implied that TT protected cells against anoxic injury and death.
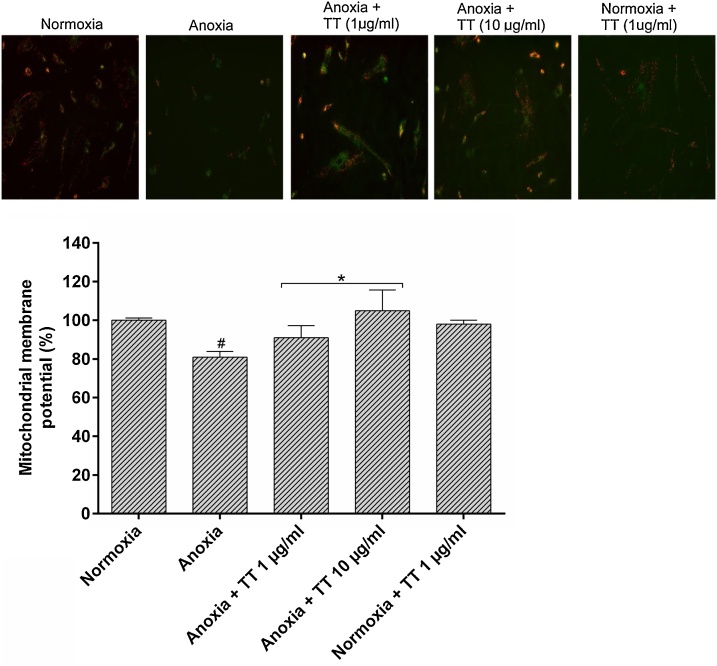
3.6. Effect of methanol fruit extract of Tetrapleura tetraptera on mitochondrial membrane potential following 3 h anoxia
There was a significant decrease (p < 0.05) in MMP of cells subjected to 3 h anoxia (severe hypoxia) compared to normoxia. This was characterized by marked staining of the “J-aggregate” form of JC- 1 (red fluorescence) with few monomer forms (green fluorescence) of normoxia-exposed cells compared to anoxia-exposed cells (Fig. 5). The difference in the loss of mitochondrial integrity of cells was visible with the loss of red stain (polarized J-aggregates form, ~ 590 nm excitation) on exposure to anoxic condition. In Fig. 5, astrocytes that were treated with 1 and 10 μg/mL TT before anoxia exposure exhibited a marked restoration of MMP (p < 0.05) in JC-1 staining compared to untreated anoxia-exposed astrocytes.
Fig. 5.
Effect of methanol fruit extract of Tetrapleura tetraptera on mitochondrial membrane potential of astrocytes. Each bar is expressed as mean ± SD (n = 3). #p < 0.05 vs normoxia; * p < 0.05 vs anoxia. TT: Methanol fruit extract of Tetrapleura tetraptera. Red stain (polarized J-aggregates form) indicated high mitochondria membrane potential while green fluorescence (monomer form) indicates depolarized (low) mitochondrial membranes. Few/loss of red stain indicated low mitochondria membrane potential.
3.7. Molecular docking
Table 3 shows that the 4 ligands (Fig. 7) possess good binding potential towards hGS. Aridanin showed the best binding potential with a minimum binding energy of -8.6 Kcal/mol, followed by naringenin (-8.3 kcal/mol), scopoletin (-7.1 kcal/mol), and ferulic acid (-6.4 kcal/mol). The binding affinity of the ligands having the lowest RMSD value (zero for upper bound and lower bound RMSD) with hGS showed a good binding pose and was considered and documented. Docking conformation was only analyzed for aridanin because unlike the other compounds, aridanin, a triterpenoid saponin, is a marker compound of Tetrapleura tetraptera and it has the best binding affinity with hGS. The result of docking analysis of aridanin from PLIP (Fig. 8) explicated its interactions with hGS through the formation of ten hydrophobic interactions with amino acid residues TRP 130, GLU 134, ALA 191, ASN 194, GLN 205, PRO 208, ASN 255 and TYR 336 (Table 4), two hydrogen bonds (H-bonds) with amino acids GLY 132 and SER 257 (Table 5) and one salt bridge formation with the amino acid residue ARG 319 (Table 6).
Table 3.
Binding affinity of some phytochemical compounds identified in Tetrapleura tetraptera methanol fruit extract with human glutamine synthetase.
| Ligand | Binding Affinity (Kcal/mol) | RMSD upper bound | RMSD lower bound | Molecular weight (g/mol) |
|---|---|---|---|---|
| Aridanin | −8.6 | 0 | 0 | 659.9 |
| Naringenin | −8.3 | 0 | 0 | 272.25 |
| Ferulic acid | −6.4 | 0 | 0 | 194.18 |
| Scopolectin | −7.1 | 0 | 0 | 192.17 |
Aridanin, a lead compound that is peculiarly isolated from Tetrapleura tetraptera fruit, has the minimum binding energy compared to other phytocompounds. The low value of RMSD (less than 1.5Ǻ, it is zero in this case) indicates the accuracy of docking. RMSD: Root mean square deviation.
Fig. 8.
(a) 3D structure of interaction between protein (hGS) and ligand (aridanin); (b) 3D structure of interaction between hGS and aridanin showing the participating amino acid residue in the protein binding pocket. In (a) and (b): structure in gold colour represent the ligand (aridanin); blue (light and deep) represent amino acid residues from the binding pocket of the protein (hGS); blue line and blue number 1 and 2 represent hydrogen bond; dash and full yellow line represents salt bridge; dash grey lines in (a) and full grey lines which are numbered 1 to 10 in (b) represents hydrophobic interactions.
Table 4.
Hydrophobic interaction between hGS and ligand (aridanin).
| S/N | Amino acid residue with position | Distance between interacting C-atom (Ǻ) | ID of ligand carbon atom | ID of protein carbon atom |
|---|---|---|---|---|
| 1 | TRP 130 | 3.34 | 3548 | 1298 |
| 2 | GLU 134 | 3.85 | 3536 | 1341 |
| 3 | ALA 191 | 3.69 | 3535 | 1862 |
| 4 | ASN 194 | 3.97 | 3529 | 1882 |
| 5 | GLN 205 | 3.52 | 3535 | 1989 |
| 6 | GLN 205 | 3.36 | 3536 | 1990 |
| 7 | PRO 208 | 3.15 | 3540 | 2016 |
| 8 | PRO 208 | 3.18 | 3548 | 2015 |
| 9 | ASN 255 | 3.33 | 3534 | 2448 |
| 10 | TYR 336 | 3.23 | 3534 | 3223 |
The hydrophobic interaction between the amino acids of protein and ligand with the geometry of interaction (distance of interacting atoms). Hydrophobic interaction stabilizes the protein-ligand (hGS-aridanin) complex. C-atom: carbon atom.
Table 5.
Hydrogen bond between amino acid residue in the binding pocket of human glutamine synthetase (hGS) and aridanin.
| S/N | Amino acid residue with position | Distance H-A (Ǻ) | Distance D-A (Ǻ) | Donor Angle | Protein donor? | Side chain? |
|---|---|---|---|---|---|---|
| 1 | GLY 132 | 3.68 | 4.10 | 107.64 | Yes | No |
| 2 | SER 257 | 2.03 | 2.98 | 170.68 | Yes | Yes |
Distance H-A: Distance between hydrogen and acceptor atoms; Distance D-A: Distance between donor and acceptor atom. Protein donor? Yes indicates protein is the donor group for the interaction. Sidechain? Yes indicates that the hydrogen bond is formed with the amino acid side chain while No indicates otherwise.
Table 6.
Salt bridge formation between the amino acid residue in the binding pocket of hGS and aridanin.
| S/N | Amino acid residue with position | Distance (Ǻ) | Protein positive | Ligand group | Ligand atom |
|---|---|---|---|---|---|
| 1 | ARG 319 | 4.37 | Yes | Carboxylate | 3555, 3556 |
The protein provides a positive charge for the interaction with the carboxylate group of the ligand to form the salt bridge.
4. Discussion
Medicinal plants are emerging as a promising option in the treatment of neurological diseases due to their pharmacologically active phytoconstituents [2,60]. Tetrapleura tetraptera has a long history of use for the traditional management of neurological diseases in West Africa. Studies have shown that its biological effects include antioxidative and anti-inflammatory properties [33,61,62] and also, its anticonvulsive [30,35] and anti-ischemic [28] properties. Polyphenols in Tetrapleura tetraptera may act synergistically against ischemia/reperfusion injury through abatement of oxidative stress, lipid peroxidation, and neurochemical dysfunctions [28].
HPLC-DAD quantification of TT revealed the presence of compounds that are pertinent in treating neurological disorders. Aridanin demonstrated anticonvulsive property by modulating nuerotransmitter and opioid receptors [35]. Cerebral infarct size in the brain of rats following cerebral ischemia was reduced by treatment with ferulic acid through antioxidative and anti-inflammatory effects [63] and naringenin regulated the expression of inflammatory molecules to give neuroprotection in rats after focal ischemia [64]. Isoliquiritigenin and scopoletin treatment protected the brain of rats following transient cerebral ischemiic injury through antioxidative, anti-inflammatory, anti-convulsive and mitochondrial modulatory effect [[65], [66], [67]].
The in vitro antioxidant and various radical scavenging activity of phytoextracts offer an insight into their potential biological effects in vivo. The antioxidant property of medicinal plants has been associated with their therapeutic potentials [6,23,68,69]. Multiple in vitro antioxidant tests such as carried out in this study are often employed to account for several antioxidant mechanisms exhibited by phytochemicals [25,40,43,70].
TT demonstrated appreciable radical scavenging and antioxidant activity notably against the highly damaging hydroxyl radical and against lipid peroxidation which is a major culprit in the etiology of many brain pathologies. The effectiveness of TT could be due to the presence of both hydrophilic and lipophilic antioxidants. The performance of TT in the various antioxidant tests with different underlying antioxidative mechanisms highlights its efficacy. Antioxidants play a vital role in reducing oxidative processes. The brain is susceptible to damage by oxidative injury and is also a target of lipid peroxidation owing to its high metabolic rate and high levels of polyunsaturated lipids [1,71,72]. As observed in this study, the lipid peroxidation inhibitory and protein anti-denaturation activities of TT shows the ability of TT to prevent oxidative damage to biomolecules. The significant antioxidant activity of TT could be related to its reduction of anoxia-induced bioenergetic disturbance in astrocytes observed in this study.
In the present study, anoxia was employed to mimic the events that leads to changes in biochemical and morphological properties of cells in the penumbra region of ischemic brain and to determine what happens to cell metabolism and viability after chronic oxygen deprivation. The astrocytes were also cultured and placed in an anoxic chamber without removing dissolved glucose. Glucose and other nutrients of cell culture were retained in the cultured media and there was no flow of new media after anoxia. In the penumbra region of the ischemic zone, cross-talk between the neurons and astrocytes is vital for the survival of neurons, so maintaining astrocyte viability is critical. Viability of astrocytes support synaptic modification and neurite outgrowth to compensate for neuron loss during ischemic insult [19,20].
Astrocytes death recorded in this study may be as a result of severe hypoxia and acidosis [56,73]. No cytotoxicity was observed after astrocytes were treated with TT suggesting that TT is not toxic to the cells at the employed concentrations. Protection of the viability and metabolic activity of astrocytes after treatment with TT and subjecting them to the anoxic condition may be due to the protective action of its bioactive components. This suggests that TT treatment may restore the function of astrocytes to provide trophic and metabolic support for neurons during metabolic brain injury.
Alteration to mitochondrial membrane integrity could cause bioenergetic disturbances and cascade of events leading to cell death [9]. Also, MMP contributes to determining the driving force for calcium entry into the mitochondria and the extent of ROS production [8]. Under hypoxic condition, the mitochondria of astrocytes like other cells, are vulnerable to the production of free radicals that leads to the reduction in ATP generation which could result in cellular damage as observed in mitochondrial dysfunction [13]. In this study, pretreatment of astrocytes with TT increased the MMP of astrocytes subjected to anoxia probably by increasing the energy capacity of the inner mitochondrial membrane and attenuating oxidative stress. Zorova et al. [9] posited that the higher the MMP, the higher the synthesis of ATP, since the energy capacity of the inner mitochondrial membrane becomes high. Therefore, a decrease in MMP will cause a reduction in mitochondrial ROS, however, this decrease must not be sustained inappropriately. Sustained inappropriate low levels of MMP could lead to reductive stress which is as detrimental as oxidative stress to mitochondrial homeostasis [74].
Needless accumulation of glutamate in the extracellular space (outside neuron, astrocytes and glia cells) is toxic to both neuronal and non-neuronal cell (excitotoxicity) and it may cause various neurological disorders if left unabated [75]. Park et al. [75] demonstrated that excitatory amino acid transporter 1, EAAC1, (not glutamate transporter 1) is the glutamate transporter responsible for transportation of glutamate in C6 cell and extracellular accumulation of β-amyloid may lead to decrease in EAAC1 expression which may result to excessive glutamate accumulation seen in Alzheimer’s disease. GS regulates excitotoxicity by converting neurotoxic glutamate (cause of excitotoxicity) to harmless glutamine with concomitant hydrolysis of ATP [15]. Molecular docking was carried out to examine the role of some of the phytochemical constituents (aridanin, ferulic acid, naringenin, and scopoletin) of TT to improve the activity of human glutamine synthetase (hGS). The binding energy of the ligands to the protein is an indicator of the stability of the ligand-protein complex [76,77]. The results indicate the ability of the ligands to improve the activity of hGS. The effectiveness of the ligands is shown by the minimum binding energy in the following order; aridanin > naringenin > scopolectin > ferulic acid.
The improved activity of the protein by ligands could further be confirmed by the formation of hydrogen bonds and hydrophobic interaction [76]. The interaction of aridanin, the marker compound in TT [35,30], with hGS was confirmed through the formation of hydrogen bonds with the formation of hydrophobic interaction and salt bridge in the neighborhood of the hydrogen bond. In this case, the side chain of the polar amino acid (SER257) participated in the hydrogen bond formation which corroborated the findings of Stojanovic and Zaric [78]. Using the following criteria; “H-A distance <2.7 Å, D-A distance <3.3 Å, D-H-A angle >90° and carbon atoms separated by <3.9 Å” SER257 is the only amino acid that is truly interacting with the ligand through hydrogen bonding, whereas, GLY132 is only interacting through hydrophobic contact [78,79]. Also, the shorter distance of the side chains to the ligand promote the formation of hydrogen bond and this may be due to the closeness of the rings of the ligand to the side chain residue (SER257) of the protein [77,78]. Therefore, the interaction of aridanin with hGS may improve its activity by positioning it to effectively condense ammonia and glutamate to form glutamine.
5. Conclusion
Methanol fruit extract of Tetrapleura tetraptera (TT) demonstrate impressive antioxidant activity through scavenging of free radical, and it is not cytotoxic. This study revealed that TT has the potential to assuage excitotoxicity, restore astrocytic viability, and reduce bioenergetic dysfunction/disturbance via its antioxidative properties in treating cerebral hypoxic conditions. This study also demonstrated that aridanin from TT could help in optimization of the activity of glutamine synthetase in order to attenuate the severity of excitotoxicity in neurological diseases. Therefore, Tetrapleura tetraptera could potentially be employed as a plant-based agent for the treatment of cerebral astrocytic hypoxia. The findings from the in vitro and in silico results in this study could be employed for further experiment in translational research.
CRediT authorship contribution statement
Ibrahim Olabayode Saliu: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Writing - review & editing. Reshma Bhagat: Validation, Formal analysis, Investigation, Writing - original draft. Olubukola Benedicta Ojo: Validation, Formal analysis, Investigation. Afolabi C. Akinmoladun: Conceptualization, Methodology, Validation, Resources, Writing - review & editing, Supervision, Project administration. M. Tolulope Olaleye: Resources, Project administration. Pankaj Seth: Conceptualization, Methodology, Resources, Supervision. Velayudhan Rema: Conceptualization, Methodology, Validation, Resources, Writing - review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
The award of DBT-TWAS sandwich postgraduate fellowship provided by the Department of Biotechnology, Government of India, and The World Academy of Science to Ibrahim O. Saliu (FR number: 3240293151) is greatly acknowledged. Thanks to Tolulope Saliu for assistance on molecular docking. This research was partly supported by NBRC core fund.
Edited by DR. A.M Tsatsaka
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2021.01.015.
Contributor Information
Ibrahim Olabayode Saliu, Email: ibrofina@gmail.com.
Afolabi C. Akinmoladun, Email: acakinmoladun@futa.edu.ng.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Sindhi V., Gupta V., Sharma K., Bhatnagar S., Kumari R., Dhaka N. Potential applications of antioxidants – a review. J. Pharm. Res. 2013;7(9):828–835. doi: 10.1016/j.jopr.2013.10.001. [DOI] [Google Scholar]
- 2.Shirley R., Ord E., Work L. Oxidative stress and the use of antioxidants in stroke. Antioxidants. 2014;3(3):472–501. doi: 10.3390/antiox3030472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhat A.H., Dar K.B., Anees S., Zargar M.A., Masood A., Sofi M.A., Ganie S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015;74:101–110. doi: 10.1016/j.biopha.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 4.Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur. J. Med. Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 5.Bhatti J.S., Bhatti G.K., Reddy P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders — A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2017;1863(5):1066–1077. doi: 10.1016/j.bbadis.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akinmoladun A.C., Saliu I.O., Olowookere B.D., Ojo O.B., Olaleye M.T., Farombi E.O., Akindahunsi A.A. Improvement of 2-Vessel occlusion cerebral ischaemia/reperfusion-induced corticostriatal electrolyte and redox imbalance, lactic acidosis and modified acetylcholinesterase activity by kolaviron correlates with reduction in neurobehavioural deficits. Ann. Neurosci. 2018;25(1):53–62. doi: 10.1159/000484517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ojo O.B., Amoo Z.A., Saliu I.O., Olaleye M.T., Farombi E.O., Akinmoladun A.C. Neurotherapeutic potential of kolaviron on neurotransmitter dysregulation, excitotoxicity, mitochondrial electron transport chain dysfunction and redox imbalance in 2-VO brain ischemia/reperfusion injury. Biomed. Pharmacother. 2019;111:859–872. doi: 10.1016/j.biopha.2018.12.144. [DOI] [PubMed] [Google Scholar]
- 8.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zorova L.D., Popkov V.A., Plotnikov E.Y., Silachev D.N., Irina B., Jankauskas S.S. Mitochondrial membrane potential. Anal. Biochem. 2018;552(01):50–59. doi: 10.1016/j.ab.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Lisa F., Blank P.S., Colonna R., Gambassi G., Silverman H.S., Stern M.D., Hansford R.G. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J. Physiol. 1995;486(1):1–13. doi: 10.1113/jphysiol.1995.sp020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vignini A. Oxidative Stress and Free Radical Damage in Neurology. Humana Press; Totowa, NJ: 2011. Stroke and oxidative stress; pp. 137–152. [DOI] [Google Scholar]
- 12.Sommer C.J. Ischemic stroke: experimental models and reality. Acta Neuropathol. 2017;133(2):245–261. doi: 10.1007/s00401-017-1667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willcox J.M., Summerlee A.J.S. Relaxin protects astrocytes from hypoxia in vitro. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0090864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeitner T.M., Battaile K., Cooper A.J.L. Critical evaluation of the changes in glutamine synthetase activity in models of cerebral stroke. Neurochem. Res. 2015;40(12):2544–2556. doi: 10.1007/s11064-015-1667-1. [DOI] [PubMed] [Google Scholar]
- 16.Vezzani A., Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology. 2015;96:70–82. doi: 10.1016/j.neuropharm.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 17.Bernstein H.G., Bannier J., Meyer-Lotz G., Steiner J., Keilhoff G., doborowonly H., Walter M., Bogerts B. Distribution of immunoreactive glutamine synthetase in the adult human and mouse brain. Qualitative and quantitative observations with special emphasis on extra-astrogial protein localization. J. Chem. Neuroanat. 2014;61–62C:35–50. doi: 10.1016/j.jchemneu.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Takano T., Oberheim N., Cotrina M.L., Nedergaard M. Astrocytes and ischemic injury. Stroke. 2009;40(1):S8–S12. doi: 10.1161/strokeaha.108.533166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steward O. 2012. Functional Neuroscience. [DOI] [Google Scholar]
- 20.Chen Y., Swanson R.A. Astrocyes and brain injury. J. Cereb. Blood Flow Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- 21.Baik S.-H., Fane M., Park J.H., Cheng Y.-L., Yang-Wei Fann D., Yun U.J. Pin1 promotes neuronal death in stroke by stabilizing Notch intracellular domain. Ann. Neurol. 2015;77(3):504–516. doi: 10.1002/ana.24347. [DOI] [PubMed] [Google Scholar]
- 22.Barthels D., Das H. Current advances in ischemic stroke research and therapies. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2018 doi: 10.1016/j.bbadis.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iqbal E., Abu K., Lim L.B.L. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J. King Saud Univ. - Sci. 2015;27(3):224–232. doi: 10.1016/j.jksus.2015.02.003. [DOI] [Google Scholar]
- 24.Procházková D., Boušová I., Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82(4):513–523. doi: 10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Gülçin İ. Antioxidant activity of food constituents: an overview I ˙ lhami Gü lçin. Arch. Toxicol. 2012;86:345–391. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- 26.Alam M.N., Bristi N.J., Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013;21(2):143–152. doi: 10.1016/J.JSPS.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oroian M., Escriche I. Antioxidants: characterization, natural sources, extraction and analysis. Food Res. Int. 2015;74:10–36. doi: 10.1016/j.foodres.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Saliu I.O., Amoo Z.A., Khan M.F., Olaleye M.T., Rema V., Akinmoladun A.C. Abatement of neurobehavioral and neurochemical dysfunctions in cerebral ischemia/reperfusion injury by Tetrapleura tetraptera fruit extract. J. Ethnopharmacol. 2020;264(113284):1–14. doi: 10.1016/j.jep.2020.113284. [DOI] [PubMed] [Google Scholar]
- 29.Kemigisha E., Owusu E.O., Elusiyan C.A., Omujal F., Tweheyo M., Bosu P.P. Tetrapleura tetraptera in Ghana, Nigeria and Uganda: households uses and local market. For. Trees Livelihoods. 2018;27(4):243–256. doi: 10.1080/14728028.2018.1498027. [DOI] [Google Scholar]
- 30.Adesina S.K., Iwalewa E.O., Johnny I.I. Tetrapleura tetraptera Taub-ethnopharmacology, chemistry, medicinal and nutritional values - a review. Br. J. Pharm. Res. 2016;12(3):1–22. doi: 10.9734/bjpr/2016/26554. [DOI] [Google Scholar]
- 31.Okwu D.E. The potentials of Ocimum gratissimum, Penrgularia extensa and Tetrapleura tetraptera as spice and flavouring agents. Nigeria Agric. J. 2004;34(1):143–148. doi: 10.4314/naj.v34i1.3184. [DOI] [Google Scholar]
- 32.Orwa C., Mutua A., Kindt R., Jamnadass R., Anthony S. 2009. Tetrapleura Tetraptera (Schum. & Thonn.) Taubert. Agroforestree Database:a Tree Reference and Selection Guide Version 4.0.http://www.worldagroforestry.org/sites/treedbs/treedatabases.asp Retrieved from. [Google Scholar]
- 33.Erukainure O.L., Onifade O.F., Odjobo B.O., Olasehinde T.A., Adesioye T.A., Tugbobo-Amisu A.O., Okonrokwo G.I. Ethanol extract of Tetrapleura tetraptera fruit peels: chemical characterization, and antioxidant potentials against free radicals and lipid peroxidation in hepatic tissues. J. Taibah Univ. Sci. 2017;11(6):861–867. doi: 10.1016/j.jtusci.2017.03.007. [DOI] [Google Scholar]
- 34.Ojewole J.A.O. Analgesic and anticonvulsant properties of Tetrapleura tetraptera (Taub) (Fabaceae) fruit aqueous extract in mice. Phytother. Res. 2005;19(12):1023–1029. doi: 10.1002/ptr.1779. [DOI] [PubMed] [Google Scholar]
- 35.Aderibigbe A.O., Iwalewa E.O., Adesina S.K., Adebanjo A.O., Ukponmwan O.E. Anticonvulsant, analgesic and hypothermic effects of aridanin isolated from Tetrapleura tetrapetra fruit in mice. J. Biol. Sci. 2007;7(8):1520–1524. doi: 10.3923/jbs.2007.1520.1524. [DOI] [Google Scholar]
- 36.Woode E., Amissah F., Duwiejua M., Fleischer T.C., Sawer I.K. Effects of tetrapleura tetraptera (Taub) fruit extract on some isolated tissues: possible mechanism(s) of antihypertensive action. J. Sci. Technol. 2008;28(1):23–34. doi: 10.4314/just.v28i1.33075. [DOI] [Google Scholar]
- 37.Odubanjo V.O., Ibukun E.O., Oboh G., Adefegha S.A. Aqueous extracts of two tropical ethnobotanicals (Tetrapleura tetraptera and Quassia undulata) improved spatial and non-spatial working memories in scopolamine-induced amnesic rats: Influence of neuronal cholinergic and antioxidant systems. Biomed. Pharmacother. 2018;99:198–204. doi: 10.1016/j.biopha.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 38.Senguttuvan J., Paulsamy S., Karthika K. Phytochemical analysis and evaluation of leaf and root parts of the medicinal herb, Hypochaeris radicata L. For in vitro antioxidant activities. Asian Pac. J. Trop. Biomed. 2014;4(Suppl. 1):S359–S367. doi: 10.12980/apjtb.4.2014c1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veskoukis A.S., Kerasioti e., Priftis A., Kouka P., Spanidis Y., Makri S., Kouretas D. A battery of translational biomarkers for the assessment of the in vitro and in vivo antioxidant action of plant polyphenolic compounds: the biomarker issue. Curr. Opin. Toxicol. 2018;13:99–109. doi: 10.1016/j.cotox.2018.10.001. [DOI] [Google Scholar]
- 40.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999;26(9–10):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 41.Adjimani J.P., Asare P. Antioxidant and free radical sacvenging activity of iron chelators. Toxicol. Rep. 2015;2:721–728. doi: 10.1016/j.toxrep.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “‘Antioxidant power’”: the FRAP assay. Anal. Biochem. 1996;239 doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 43.Apak R., Güçlü K., Özyürek M., Çelik S.E. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta. 2008;160(4):413–419. doi: 10.1007/s00604-007-0777-0. [DOI] [Google Scholar]
- 44.Sakat S.S., Juvekar A.R., Gambhire M.N. Invitro antioxidant and antiinflammatory activity of methanol extract of oxalis corniculata linn. Int. J. Pharm. Pharm. Sci. 2010;2(1):146–155. [Google Scholar]
- 45.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 46.Devasagayam T.P.A., Boloor K.K., Ramasarma T. Minireview Methods for estimating lipid peroxidation: an analysis of merits and demerits. Indian J. Biochem. Biophys. 2003;40:300–308. [PubMed] [Google Scholar]
- 47.Fatima M., Prajapati B., Saleem K., Kumari R., Singal C., Seth P. Novel insight into role of miR-320a-VDAC1 axis in astrocyte-mediated neuronal damage in neuroAIDS. GLIA. 2017;65(2):250–263. doi: 10.1002/glia.23089. [DOI] [PubMed] [Google Scholar]
- 48.Bhagat R., Prajapati B., Narwal S., Agnihotri N., Adlakha Y.K., Sen J., mani S., Seth P. Zika virus E protein alters properties of human fetal neural stem cells by modulating microRNA circuitry. Cell Death Diff. 2018;25(10):1837–1854. doi: 10.1038/s41418-018-0163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 50.Berridge M.V., Herst P.M., Tan A.S. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol. Ann. Rev. 2005;11:127–152. doi: 10.1016/S1387-2656(05)11004-7. [DOI] [PubMed] [Google Scholar]
- 51.Korenic A., Boltze J., Deten A., Peters M., Andjus P., Radenovic L. Astrocytic mitochondrial membrane hyperpolarization following extended oxygen and glucose deprivation. PLoS One. 2014;9(2):1–7. doi: 10.1371/journal.pone.0090697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xing C., Arai K., Lo E.H., Hommel M. Pathophysiologic cascades in ischemic stroke. Int. J. Stroke. 2012;7(5):378–385. doi: 10.1111/j.1747-4949.2012.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inose Y., Kato Y., Kitagawa K., Uchiyama S., Shibata N. Activated microglia in ischemic stroke penumbra upregulate MCP-1 and CCR2 expression in response to lysophosphatidylcholine derived from adjacent neurons and astrocytes. Neuropathology. 2015;35(3):209–223. doi: 10.1111/neup.12182. [DOI] [PubMed] [Google Scholar]
- 54.Meadows K.L. Experimental models of focal and multifocal cerebral ischemia: A review. Rev. Neurosci. 2018;29(6):661–674. doi: 10.1515/revneuro-2017-0076. [DOI] [PubMed] [Google Scholar]
- 55.Wu M.Y., Yiang G.T., Liao W.T., Tsai A.P.Y., Cheng Y.L., Cheng P.W. Current mechanistic concepts in ischemia and reperfusion injury. Cell. Physiol. Biochem. 2018;46(4):1650–1667. doi: 10.1159/000489241. [DOI] [PubMed] [Google Scholar]
- 56.Swanson R.A., Farrell K., Simon R.P. Acidosis causes failure of astrocyte glutamate uptake during hypoxia. J. Cereb. Blood Flow Metab. 1995;15(3):417–424. doi: 10.1038/jcbfm.1995.52. [DOI] [PubMed] [Google Scholar]
- 57.Lai T.W., Zhang S., Wang Y.T. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Progress Neurobiol. 2014;115:157–188. doi: 10.1016/j.pneurobio.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 58.Krajewski W.W., Collins R., Holmberg-Schiavone L., Jones T.A., Karlberg T., Mowbray S.L. Crystal structures of mammalian glutamine synthetases illustrate substrate-induced conformational changes and provide opportunities for drug and herbicide design. J. Mol. Biol. 2008;375:217–228. doi: 10.2210/pdb2ojw/pdb. [DOI] [PubMed] [Google Scholar]
- 59.Salentine S., Schreiber S., Haupt V.J., Adasme M.F., Schroeder M. PLIP: fully automated protein-ligand interation profiler. Nucleic Acids Res. 2015;43(W1):W443–W447. doi: 10.1093/nar/gkv315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajendran P., Nandakumar N., Rengarajan T., Palaniswami R., Gnanadhas E.N., Lakshminarasaiah U., Nishigaki I. Antioxidants and human diseases. Clin. Chim. Acta. 2014;436:332–347. doi: 10.1016/j.cca.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 61.Ojewole J.A.O., Adewunmi C.O. Anti-inflammatory and hypoglycaemic effects of Tetrapleura tetraptera (Taub) [fabaceae] fruit aqueous extract in rats. J. Ethnopharmacol. 2004;95(2–3):177–182. doi: 10.1016/j.jep.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 62.Moukette B.M., Pieme A.C., Biapa P.C.N., Njimou J.R., Stoller M., Bravi M., Yonkeu Ngogang J. In vitro ion chelating, antioxidative mechanism of extracts from fruits and barks of tetrapleura tetraptera and their protective effects against fenton mediated toxicity of metal ions on liver homogenates. Evid.-Based Complement. Altern. Med. 2015;2015:1–14. doi: 10.1155/2015/423689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen C., HO T., Lee E., Su S., Tang N., Hsieh C. Ferulic acid reduces cerebral infarct through its antioxidative and anti-inflammatory effects following transient focal cerebral ischemia in rats. Am. J. Chin. Med. 2008;36(6):1105–1119. doi: 10.1142/S0192415X08006570. [DOI] [PubMed] [Google Scholar]
- 64.Bai X., Zhang X., Chen L., Zhang J., Zhang L., Zhao X. Protective effect of naringenin in experimental ischemic stroke: down-regulated NOD2, RIP2, NF-kB, MMP-9 and up-regulated claudin-5 expression. Neurochem. Res. 2014;39(8):1405–1415. doi: 10.1007/s11064-014-1326-y. [DOI] [PubMed] [Google Scholar]
- 65.Venugopala K., Rashmi V., Odhav B. Review on natural Coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013:1–14. doi: 10.1155/2013/963248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Connell B.J., Saleh M.C., Rajagopal D., Saleh T.M. UPEI-400, a conjugate of lipoic acid and scopoletin, mediates neuroprotection in a rat model of ischemia/reperfusion. Food Chem. Toxicol. 2017;100:175–182. doi: 10.1016/j.fct.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 67.Ramalingam M., Kim H., Lee Y., Yun-Il L. Phytochemical and pahrmacological role of Liquiritigenin and Isoliquiritigenin from Radix glycyrrhizae in human health and disease models. Front. Aging Neurosci. 2018;10:348. doi: 10.3389/fnagi.2018.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Falode J.A., Akinmoladun A.C., Olaleye M.T. Ameliorative property of Kigelia africana crude and flavonoid leaf extracts on aluminum-induced hepatotoxicity in albino rats. Comp. Clin. Pathol. 2019;28(5):1495–1506. doi: 10.1007/s00580-019-03004-y. [DOI] [Google Scholar]
- 69.Olugbodi J.O., Tincho M.B., Oguntibeju O.O., Olaleye M.T., Akinmoladun A.C. Glyphaea brevis – in vitro antioxidant and in silico biological activity of major constituents and molecular docking analyses. Toxicol. Vitro. 2019;59:187–196. doi: 10.1016/j.tiv.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 70.Gan R.Y., Xu X.R., Song F.L., Kuang L., Li H.B. Antioxidant activity and total phenolic content of medicinal plants associated with prevention and treatment of cardiovascular and cerebrovascular diseases. J. Med. Plant Res. 2010;4:2438–2444. [Google Scholar]
- 71.Akinmoladun C.A., Crown O.O., Ojo B.O., Olaleye M.T., Farombi O.E. Antidenaturation and antioxidative properties of phytochemical components from Spondias mombin. Afr. J. Biochem. Res. 2014;8(5):101–110. doi: 10.5897/ajbr2014.0769. [DOI] [Google Scholar]
- 72.Obade E., Ilesanmi O.B., Crown O., Akinmoladun A.C., Olaleye T.M., Akindahunsi A.A. Neuromodulatory effect of solvent fractions of Africa eggplant (Solanium dadyphyllum) against KCN-induced mitochondria damage, viz. NADH-succinate dehydrogenase, NADH- cytochrome c reductase, and succinate-cytochrome c reductase. Clin. Phytosci. 2018;4(1) doi: 10.1186/s40816-018-0068-9. [DOI] [Google Scholar]
- 73.Danilov C.A., Fiskum G. Hyperoxia promotes astrocyte cell death after oxygen and glucose deprivation. GLIA. 2008;56(7):801–808. doi: 10.1002/glia.20655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zorov D.B., Kinnally K.W., Tedeschi H. Voltage activation of heart inner mitochondrial membrane channels. J. Bioenerget. Biomembr. 1992;24:119–124. doi: 10.1007/bf00769538. [DOI] [PubMed] [Google Scholar]
- 75.Park S.-H., Lee J.-Y., Jhee K.-H., Yang S.-A. Amyloid-β peptides inhibit the expression of AQP4 and glutamate transporter EAAC1 in insulin-treated C6 glioma cells. Toxicol. Rep. 2020;7:1083–1089. doi: 10.1016/j.toxrep.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahmad S., Nadeem H., Muhammad S.A., Naz S., Imran M., Saeed A. Synthesis, antimicrobial and α-glucosidase inhibitory potential of mannich bases of mercapto oxadiazoles and their molecular docking studies. Farmacia. 2018;66(4):708–717. doi: 10.31925/farmacia.2018.4.22. [DOI] [Google Scholar]
- 77.Bhatia A., Singh B., Arora R., Arora S. In vitro evaluation of the α-glucosidase inhibitory potential of methanolic extracts of traditionally used antidiabetic plants. BMC Complem. Altern. Med. 2019;19(1):1–9. doi: 10.1186/s12906-019-2482-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stojanovic S.D., Zaric S.D. Hydrogen bonds and hydrophobic interactions of porphyrins in porphyrin-containing proteins. Open Struct. Biol. J. 2009;3(1):34–41. doi: 10.2174/1874199100903010034. [DOI] [Google Scholar]
- 79.Sherman W., Tidor B. Novel method for probing the specificity binding profile of ligands: applications to HIV protease. Chem. Biol. Drug Des. 2008;71:387–407. doi: 10.1111/j.1747-0285.2008.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]